- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information

6 Enzyme Activity - Biochem
Biochemistry (nr - chm 110), de la salle medical and health sciences institute.
Recommended for you
Students also viewed.
- Long Test II - Notes
- Vitamins-AND- Minerals
- 1 The Human Organism - none
- Sociological Perspectives Introduction to Sociology
- 3 Lipids (Folch Method)
- What Is a Global City Newgeography
Related documents
- 2 Characterization of Carbohydrates
- Mills The-Promise - Biochem
- 5 Urinalysis - Biochem
- Experiment 2 Post-Lab Report
- Protein Post-Lab
Preview text
BIOCHEMISTRY LABORATORY MANUAL
EXPERIMENT NO. 6
Factors affecting enzyme activity, i. background.
Enzymes are proteins that act as biological catalysts. They are highly efficient and increase the rate of the reaction by as much as 10 9 faster. Enzymes bind to their substrate, the substance that they act upon, via noncovalent interactions to form an enzyme-substrate complex. The enzyme will process the substrate to form the products. The products are released, and the enzyme can be used for another reaction.
The activity of the enzyme can be enhanced by changing the enzyme concentration, substrate concentration, and the temperature and pH conditions. For every enzyme, there is an optimum temperature and pH, depending on the natural environment of the enzyme. At these conditions, the enzyme has maximum activity. Below or above these conditions, the activity of the enzyme decreases. For example, pepsin, an enzyme found in the stomach, has an optimum pH of 2.
In this lab, we will study the properties of a-amylase, an enzyme found in saliva. This enzyme is responsible for hydrolyzing the a1-4 glycosidic linkages found in carbohydrates, such as starch, into smaller sugar units, such as disaccharides and oligosaccharides.
II. OBJECTIVES
At the end of this activity, the students should be able to: 1. Study the effect of enzyme concentration, temperature, and pH on the enzyme’s activity 2. Determine the enzyme’s optimum temperature and pH
III. MATERIALS
A. The students need to bring the following: None
B. The instructor/technician/custodian should prepare the following:
Reagents Prepared buffers from Experiment # 0 M Acetate buffer (pH 4 and 5) 0 M Phosphate buffer (pH 6 and 8) 0 M Bicarbonate buffer (pH 10)
1% starch solution Iodine solution
Instrument Temperature-controlled water bath
C. The students need to borrow the following from the stockroom: Item Qty. Item Qty. 100 mL beaker 1 Spot plate 1 Test tubes 10 10-mL Graduated cylinder 1 50-mL Graduated cylinder 1 Pasteur pipette 2 IV. SAFETY
- Wear lab gown and protective goggles at all times.
- Place highly volatile samples under fume hood.
- Wash hands thoroughly with soap and water before leaving the laboratory.
- Incubate test tubes 1-10 in a 37°C water bath.
- While waiting, place 2 drops of iodine solution on each well on a spot plate.
- After the 10 minute incubation, mix test tube 1 and 6 together and immediately place 3 drops of this mixture onto one well on the spot plate. This will be your zero (0) minute. Record your observations.
- After 1 minute, take another 3 drops from the mixture and place onto the next well on the spot plate. This will be your one (1) minute. Record your observations.
- Repeat step 8 until you get a yellow-colored solution on the spot plate.
- For your next run, combine test tube 2 and 7 and repeat steps 7-9.
- Repeat the steps for the following combinations: Test tube 3 and 8 Test tube 4 and 9 Test tube 5 and 10
- Plot pH vs. 1/time. Determine the optimum pH from your plot.
WASTE DISPOSAL: Dispose all wastes in the Organic Waste Bottle.
DATA REPORT SHEET – EXPERIMENT NO. 6
Enzyme activity, name: class no.:, section: date performed:, instructor: date completed:.
A. Effect of Enzyme Concentration
Test Tube No.
Dilution Volume of Saliva (mL)
Volume of Phosphate Buffer (pH 6) (mL) 1 0 1 mL from the stock 0
2 1:10 ____ from Tube ____
3 1:100 ____ from Tube ____
4 1:1000 ____ from Tube ____
Show your work below for Test Tubes 2-4.
Instructor’s Initials _____
B. Effect of Temperature
Temperature (°C) Observations 1 + 4
Test tube 5 + 10
pH ______ Time (min) Observations
POST-LAB QUESTIONS – EXPERIMENT NO. 6
In Part A, does the activity of the enzyme increase or decrease when the amount of the enzyme is increased? Explain.
In Part B, what is the optimal temperature for the enzyme?
In Part B, why should we add a buffer to all of the test tubes?
In Part C, what is the optimal pH of the enzyme?
In Part C, why should we incubate all of the the test tubes at 37°C?
- Multiple Choice
Course : Biochemistry (NR - CHM 110)
University : de la salle medical and health sciences institute.

- Discover more from: Biochemistry NR - CHM 110 De La Salle Medical and Health Sciences Institute 79 Documents Go to course
- More from: Biochemistry NR - CHM 110 De La Salle Medical and Health Sciences Institute 79 Documents Go to course
Investigation: Enzymes

Enzyme Lab Teacher's Guide
Measure the effects of changes in temperature, pH, and enzyme concentration on reaction rates of an enzyme
Explain how environmental factors affect the rate of enzyme-catalyzed reactions.
INTRODUCTION: What would happen to your cells if they made a poisonous chemical? You might think that they would die. In fact, your cells are always making poisonous chemicals. They do not die because your cells use enzymes to break down these poisonous chemicals into harmless substances.
Enzymes are proteins that speed up the rate of reactions that would otherwise happen more slowly. The enzyme is not altered by the reaction. You have hundreds of different enzymes in each of your cells.
Each of these enzymes is responsible for one particular reaction that occurs in the cell. In this lab, you will study an enzyme that is found in the cells of many living tissues. The name of the enzyme is catalase; it speeds up a reaction which breaks down hydrogen peroxide, a toxic chemical, into 2 harmless substances--water and oxygen. Light can also break down H 2 O 2 which is why the chemical is sold in dark containers.
The reaction is: 2H 2 O 2 → 2H 2 O + O 2
This reaction is important to cells because hydrogen peroxide is produced as a byproduct of many normal cellular reactions. If the cells did not break down the hydrogen peroxide, they would be poisoned and die. In this lab, you will study the catalase found in liver cells. You will be using chicken or beef liver. It might seem strange to use dead cells to study the function of enzymes. This is possible because when a cell dies, the enzymes remain intact and active for several weeks, as long as the tissue is kept refrigerated.
MATERIALS: 6 Test tubes / Test tube holders 3% Hydrogen peroxide Ice / Hot water Straight-edged razor blade Scissors and Forceps Measuring Pipettes Stirring rod
Fresh liver, Apple, and Potato, Yeast Vinegar / Baking Soda HCL and NaOH pH paper (optional)
PART A - Observe Normal Catalase Reaction
1. Place about 2 ml of the 3% hydrogen peroxide solution into a clean test tube.
2. Using forceps and scissors cut a small piece of liver and add it to the test tube. Push it into the hydrogen peroxide with a stirring rod. Observe the bubbles. What gas is being released? (consider the equation above) _____
Throughout this investigation you will estimate the rate of the reaction (how rapidly the solution bubbles) on a scale of 0-5 (0=no reaction, 1=slow, ..... 5= very fast). Assume that the reaction in step 2 proceeded at a rate of "4"
Recall that a reaction that absorbs heat is endothermic; a reaction that gives off heat is exothermic. Feel the temperature of the test tube with your hand.
Has it gotten warmer or colder? Is the reaction endothermic or exothermic?
3. Pour off the liquid into a second test tube. Assuming the reaction is complete, what is this liquid composed of?
What do you think would happen if you added more liver to this liquid?
Test this and record the reaction rate. Reaction Rate:
4. Add another 2ml of hydrogen peroxide to the liver remaining in the first test tube. What is the reaction rate?
Synthesis -- Answer the question: Is catalase reusable?
REASONING .
Part B - What Tissues Contain Catalase?
You will now test for the presence of catalase in tissues other than liver. Place 2 ml of hydrogen peroxide in each of 3 clean test tubes and then add each of the three test substances to the tubes. As you add each test substance, record the reaction rate (0-5) for each tube.

Synthesis -- Do all living tissues contain catalase? Claim:
Evidence: Reasoning:
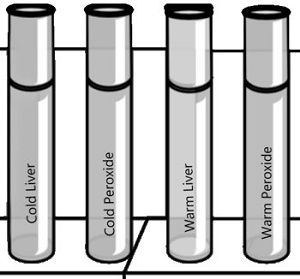
PART C - What is the Effect of Temperature on Catalase Activity?
1. Put a piece of liver into the bottom of a clean test tube and cover it with a small amount of water. Place this test tube in a boiling water bath for 5 minutes.
2. Remove the test tube from the hot water bath, allow it to air cool, then pour out the water. Add 2 ml of hydrogen peroxide. CAUTION: Use a test-tube holder for hot test tubes.
What is the reaction rate for the boiled liver and peroxide? __________
3. Put equal quantities of liver into 2 clean test tubes and 1 ml H 2 O 2 into 2 other test tubes. Put one test tube of liver and one of H 2 O 2 into an ice bath. Place the other set in a warm water bath (not boiling).
After 3 minutes, pour each tube of H 2 O 2 into the corresponding tube of liver and observe the reaction
What is the reaction rate for the cold liver/peroxide? _____ What is the reaction rate for the warm liver/peroxide? ____
Synthesis -- How does temperature affect the catalase enzyme?
Claim:
PART D - What is the Effect of pH on Catalase Activity
1. Add 2 ml hydrogen peroxide to 4 clean test tubes, then add:
Tube 1 – add 3 drops of acetic acid (vinegar) pH =_______ Tube 2 – add 3 drops of sodium bicarbonate (base) pH =______ Tube 3 – add 3 drops of water (neutral) pH =_____ Tube 4 -- add 3 drops of 1M NaOH pH = _____
Now add liver to each of the test tubes (try to do it all at about the same time, so you can easily compare)
Rate of Reaction for:
Strong Acid (HCL) ____ Acid _____ Neutral ______ Base_____ Strong base (NAOH) _____
1. How does pH affect the reaction rate of catalase? Propose a way to refine your experiment to find the exact , or OPTIMAL pH and temperature of catalase.
2. The following graph shows reaction rates of various enzymes in the body. Pepsin is found in the stomach, amylase in the saliva, and phosphatase in the liver.
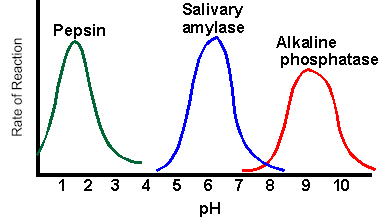
Synthesis: How does pH affect the activity of enzymes?
Claim:

Part E - Design an Experiment
Lactaid is a product designed to help people who cannot digest milk sugar (lactose) because they are missing the enzyme lactase. Many people are lactose-intolerant, a condition that is mainly genetic. Lactase breaks down lactose into two subunits: glucose and galactose.
To test for the presence of monosaccharides and reducing disaccharide sugars in food, the food sample is dissolved in water, and a small amount of Benedict's reagent is added. The solution should progress in the colors of blue (with no glucose present), green, yellow, orange, red, and then brick red when there is a large amount of glucose present. (Google benedict's test to see the way this looks.)
Design an experiment where you would determine how quicly lactaid works to break down milk sugar at different temperatures.. Be specific in your description, use drawings if necessary.
Other Resources on Enzymes
Analyzing Graphics - Enzymes - shows substrates and enzyme interactions and explores competitive inhibition
Observe Catalase Activity in Yeast - create sodium alginate spheres to observe how catalase breaks down hydrogen peroxide
Enzyme Activity Using Toothpickase - simulate the activity of an enzyme by breaking toothpicks.

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
7 Lab 6. Enzymes – Catalase Function
Lab 6: enzymes – catalase function.
- Practice and apply hypothesis testing.
- Practice experimental design.
- Gain a better understanding of enzymes and some conditions that affect enzyme activity and the rate of an enzyme-catalyzed reaction.
- Understand these terms: enzyme, enzyme activity, active site, substrate, enzyme-substrate complex, product, denature, competitive inhibition, and noncompetitive inhibition.
INTRODUCTION
Enzymes are proteins that increase the rate of chemical reactions. This process is called catalysis . All cells use enzymes for metabolism , the sum of all chemical and physical reactions that a cell uses to break down nutrients to produce energy and to build up important molecules needed for cell function. Enzymes increase the rate of a reaction by lowering activation energy but are not themselves consumed in the reaction. Thus, one enzyme can catalyze repeated rounds of the same reaction.
As with all proteins, an enzyme’s function depends completely on its shape. Enzymes have a very complex three-dimensional structure consisting of one or more polypeptide chains folded to form an active site – a specific area into which the substrate (material to be acted on by the enzyme) will fit. The shape of the enzyme determines which reaction it will catalyze, because only one substrate will fit into its active site.

Changes in temperature, pH, and/or the addition of certain ions or molecules may affect the structure of an enzyme’s active site and thus the ability of the enzyme to catalyze the reaction (the enzyme’s “enzyme activity”). Changing an enzyme’s shape so that it is no longer biologically active is called denaturation . Enzyme inhibitors can occupy the active site (competitive inhibitors) or change the shape of the active site by binding elsewhere (noncompetitive inhibitors). Hence, these factors also affect the rate of the reaction in which the enzyme participates. The rate of an enzymatic reaction can also be affected by the relative concentrations of enzyme and substrate.
Salt concentration: If the salt concentration is very low or zero, the charged amino acid side-chains of the enzyme will stick together. The enzyme will denature and form an inactive precipitate. If, on the other hand, the salt concentration is very high, normal interaction of charged groups will be blocked, new interactions occur, and again the enzyme will precipitate. An intermediate salt concentration such as that of blood (0.9%) or cytoplasm is optimum for most enzymes.
pH: pH is a logarithmic scale that measures the acidity or H+ concentration in a solution. As the pH is lowered, an enzyme will tend to gain H+ ions, and eventually enough side chains will be affected so that the enzyme’s shape is disrupted. Likewise, as the pH is raised, the enzyme will lose H+ ions and eventually lose its active shape. Many enzymes have an optimum in the neutral pH range and are denatured at either extremely high or low pH. Some enzymes, such as those which act in the human stomach where the pH is very low, will have an appropriately low pH optimum. A buffer is a compound that will gain or lose H+ ions so that the pH changes very little.
Temperature: All chemical reactions speed up as the temperature is raised. As the temperature increases, more of the reacting molecules have enough kinetic energy to undergo the reaction. Since enzymes are catalysts for chemical reactions, enzyme reactions also tend to go faster with increasing temperature. However, if the temperature of an enzyme-catalyzed reaction is raised still further, an optimum is reached: above this point the kinetic energy of the enzyme and water molecules is so great that the structure of the enzyme molecules starts to be disrupted. The positive effect of speeding up the reaction is now more than offset by the negative effect of denaturing more and more enzyme molecules. Many proteins are denatured by temperatures around 40-50C, but some are still active at 70-80C, and a few even withstand being boiled.
In this exercise you will study the enzyme catalase, which accelerates the breakdown of hydrogen peroxide (a common end product of oxidative metabolism) into water and oxygen, according to the summary reaction:
2H 2 O 2 + Catalase —-> 2H 2 O + O 2 + Catalase
Catalase is found in animal and plant tissues, and is especially abundant in plant storage organs such as potato tubers, corms, and in the fleshy parts of fruits. You will isolate catalase from potato tubers and measure its rate of activity under different conditions.
The fact that one of the products of the above reaction (oxygen) forms a gas, it is convenient to observe the conversion from reactants to products. A small piece of filter paper can be soaked in a solution of catalase and immersed in a solution of hydrogen peroxide. It will immediately sink to the bottom of the vessel. As the catalase converts the hydrogen peroxide to water and oxygen, some of the oxygen gas accumulates on the disk, changing buoyancy of the disk and floating to the surface. The elapsed time from immersion to floatation is thus proportional to the rate of the reaction (the time required to produce sufficient product (O 2 ) to float the disk). This allows us to explore the effect of a number of variables on the rate of a reaction.

Part A : Extraction of Catalase
- Peel a fresh potato tuber and cut the tissue into small cubes. Weigh out 50 g of tissue.
- Place the tissue, 50 ml of cold distilled water, and a small amount of crushed ice in a pre-chilled blender.
- Homogenize for 30 seconds at high speed. From this point on, the enzyme preparation must be carried out in an ice bath.
- Filter the potato extract, then pour the filtrate into a 100-ml graduated cylinder. Add cold distilled water to bring up the final volume to 100 ml. Mix well. This extract will be arbitrarily labeled 100 units of enzyme per ml (100 units/ml). (NOTE – this will be enough catalase for all lab groups)
Work in groups of 4.
- Tape or permanent marker for labeling
- Five 50 ml beakers or large test tubes.
- Stock 3% H 2 O 2
- Catalase solution
- No2 Whatman filter paper
- Hole punchers
- Beaker with water
- Water baths at the following temperatures (4, 25, 37, 60 o C). These will be beakers on hot plates with temperature monitored by a thermometer.
PART B: Effect of Substrate Concentration
Before beginning, make a hypothesis and prediction about the scientific questions we are asking.
Q1. How does substrate concentration affect the speed at which catalase converts H 2 O 2 into water and oxygen?
Ha (Alternative hypothesis); H0 (Null hypothesis)
Write your scientific prediction (remember to use if—then—).
What are independent and dependent variables in this experiment?
- Place 20 ml stock 3% H 2 O 2 in one of the beakers or test tubes and label. Make sure your label identifies your group and concentration of H 2 O 2 .
- Dilute stock 3% H 2 O 2 to make 1.5%, 0.75%, 0.38% and 0.19%. You should figure out how to do this by serial dilution of your 3% H 2 O 2 stock. All beakers or test tubes should contain 10 ml except for the last beaker containing 0.19% H 2 O 2 .
- Make 25 small filter paper disks with a hole punch.
- With a forceps, grab a disk and immerse it in catalase solution for 3 seconds. BE PRECISE WITH THE TIME IT IS IMMERSED IN THE ENZYME. Immediately drop it into one of the solutions of H 2 O 2 and start your timer. If you look closely, you will see bubbles of O 2 forming on the disk. Stop your timer the moment the paper disk rises to the surface. Remove the paper disk and repeat the process four more times.
- Repeat step 4 for each of the concentration of H 2 O 2 .
- Record your data in a table.
DATA ANALYSIS
Graph your results by hand in your lab notebook. Plot the independent variables on X axis, dependent variable on Y axis. Use the averages of your replicates for the graph.
PART C: The effect of temperature on enzyme function and the reaction
Before beginning, make some hypothesis and predictions about the scientific questions we are asking.
Q1. How does temperature affect the speed at which catalase converts hydrogen peroxide into water and oxygen?
Ha (Alternative hypothesis)
H0 (Null hypothesis)
Write your scientific prediction here (remember to use if—then—)
- Prepare 5 test tubes with 10 ml each of 1.5% H 2 O 2 solution, and label them to identify your group and temperature. Keep one of your test tubes immersed in water at room temperature, the others in water baths at 4 o C (on ice), 37 o C, and 60 o C, respectively.
- Make 25 more filter paper disks.
- Measure the reaction rate as you did in part 1, by dipping each filter paper into catalase for 3 seconds, then recording the time it takes for a disk to rise to the top of each test tube after dropped to the bottom. As in part 1, repeat this procedure with 5 filter paper per test tube.
Post-lab Questions – Answer these questions in your lab notebook
- What were your conclusions regarding the effect of substrate concentration on catalase activity?
- What were your conclusions regarding the effect of temperature on catalase activity? Identify the optimum temperature on catalase activity, in other words, at what temperature was the amount of product produced by the enzyme-catalyzed reaction greatest ?
- If an enzyme were isolated from an organism, such as a clam, that lived in seawater that averages 14°C, what would you predict would be the optimal temperature for that enzyme, and why?
- Define enzyme denaturation in terms of protein structure.
LWTech General Biology (BIOL&160) Lab Protocols Copyright © by Lake Washington Institute of Technology is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book
Practical Biology
A collection of experiments that demonstrate biological concepts and processes.

Observing earthworm locomotion

Practical Work for Learning

Published experiments
Investigating an enzyme-controlled reaction: catalase and hydrogen peroxide concentration, class practical or demonstration.
Hydrogen peroxide ( H 2 O 2 ) is a by-product of respiration and is made in all living cells. Hydrogen peroxide is harmful and must be removed as soon as it is produced in the cell. Cells make the enzyme catalase to remove hydrogen peroxide.
This investigation looks at the rate of oxygen production by the catalase in pureed potato as the concentration of hydrogen peroxide varies. The oxygen produced in 30 seconds is collected over water. Then the rate of reaction is calculated.
Lesson organisation
You could run this investigation as a demonstration at two different concentrations, or with groups of students each working with a different concentration of hydrogen peroxide. Individual students may then have time to gather repeat data. Groups of three could work to collect results for 5 different concentrations and rotate the roles of apparatus manipulator, result reader and scribe. Collating and comparing class results allows students to look for anomalous and inconsistent data.
Apparatus and Chemicals
For each group of students:.
Pneumatic trough/ plastic bowl/ access to suitable sink of water
Conical flask, 100 cm 3 , 2
Syringe (2 cm 3 ) to fit the second hole of the rubber bung, 1
Measuring cylinder, 100 cm 3 , 1
Measuring cylinder, 50 cm 3 , 1
Clamp stand, boss and clamp, 2
Stopclock/ stopwatch
For the class – set up by technician/ teacher:
Hydrogen peroxide, range of concentrations, 10 vol, 15 vol, 20 vol, 25 vol, and 30 vol, 2 cm 3 per group of each concentration ( Note 1 )
Pureed potato, fresh, in beaker with syringe to measure at least 20 cm 3 , 20 cm 3 per group per concentration of peroxide investigated ( Note 2 )
Rubber bung, 2-holed, to fit 100 cm 3 conical flasks – delivery tube in one hole (connected to 50 cm rubber tubing)
Health & Safety and Technical notes
Wear eye protection and cover clothing when handling hydrogen peroxide. Wash splashes of pureed potato or peroxide off the skin immediately. Be aware of pressure building up if reaction vessels become blocked. Take care inserting the bung in the conical flask – it needs to be a tight fit, so push and twist the bung in with care.
Read our standard health & safety guidance
1 Hydrogen peroxide: (See CLEAPSS Hazcard) Solutions less than 18 vol are LOW HAZARD. Solutions at concentrations of 18-28 vol are IRRITANT. Take care when removing the cap of the reagent bottle, as gas pressure may have built up inside. Dilute immediately before use and put in a clean brown bottle, because dilution also dilutes the decomposition inhibitor. Keep in brown bottles because hydrogen peroxide degrades faster in the light. Discard all unused solution. Do not return solution to stock bottles, because contaminants may cause decomposition and the stock bottle may explode after a time.
2 Pureed potato may irritate some people’s skin. Make fresh for each lesson, because catalase activity reduces noticeably over 2/3 hours. You might need to add water to make it less viscous and easier to use. Discs of potato react too slowly.
3 If the bubbles from the rubber tubing are too big, insert a glass pipette or glass tubing into the end of the rubber tube.
SAFETY: Wear eye protection and protect clothing from hydrogen peroxide. Rinse splashes of peroxide and pureed potato off the skin as quickly as possible.
Preparation
a Make just enough diluted hydrogen peroxide just before the lesson. Set out in brown bottles ( Note 1 ).
b Make pureed potato fresh for each lesson ( Note 2 ).
c Make up 2-holed bungs as described in apparatus list and in diagram.
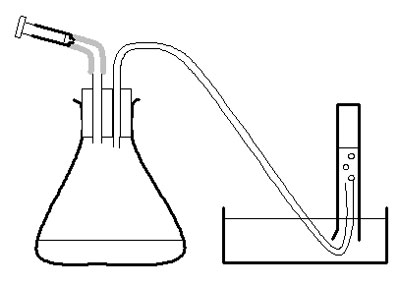
Investigation
d Use the large syringe to measure 20 cm 3 pureed potato into the conical flask.
e Put the bung securely in the flask – twist and push carefully.
f Half-fill the trough, bowl or sink with water.
g Fill the 50 cm 3 measuring cylinder with water. Invert it over the trough of water, with the open end under the surface of the water in the bowl, and with the end of the rubber tubing in the measuring cylinder. Clamp in place.
h Measure 2 cm 3 of hydrogen peroxide into the 2 cm 3 syringe. Put the syringe in place in the bung of the flask, but do not push the plunger straight away.
i Check the rubber tube is safely in the measuring cylinder. Push the plunger on the syringe and immediately start the stopclock.
j After 30 seconds, note the volume of oxygen in the measuring cylinder in a suitable table of results. ( Note 3 .)
k Empty and rinse the conical flask. Measure another 20 cm 3 pureed potato into it. Reassemble the apparatus, refill the measuring cylinder, and repeat from g to j with another concentration of hydrogen peroxide. Use a 100 cm 3 measuring cylinder for concentrations of hydrogen peroxide over 20 vol.
l Calculate the rate of oxygen production in cm 3 /s.
m Plot a graph of rate of oxygen production against concentration of hydrogen peroxide.
Teaching notes
Note the units for measuring the concentration of hydrogen peroxide – these are not SI units. 10 vol hydrogen peroxide will produce 10 cm 3 of oxygen from every cm 3 that decomposes.( Note 1 .)
In this procedure, 2 cm 3 of 10 vol hydrogen peroxide will release 20 cm 3 of oxygen if the reaction goes to completion. 2 cm 3 of liquid are added to the flask each time. So if the apparatus is free of leaks, 22 cm 3 of water should be displaced in the measuring cylinder with 10 vol hydrogen peroxide. Oxygen is soluble in water, but dissolves only slowly in water at normal room temperatures.
Use this information as a check on the practical set-up. Values below 22 cm 3 show that oxygen has escaped, or the hydrogen peroxide has not fully reacted, or the hydrogen peroxide concentration is not as expected. Ask students to explain how values over 22 cm 3 could happen.
An error of ± 0.05 cm 3 in measuring out 30 vol hydrogen peroxide could make an error of ± 1.5 cm 3 in oxygen production.
Liver also contains catalase, but handling offal is more controversial with students and introduces a greater hygiene risk. Also, the reaction is so vigorous that bubbles of mixture can carry pieces of liver into the delivery tube.
If collecting the gas over water is complicated, and you have access to a 100 cm 3 gas syringe, you could collect the gas in that instead. Be sure to clamp the gas syringe securely but carefully.
The reaction is exothermic. Students may notice the heat if they put their hands on the conical flask. How will this affect the results?
Health and safety checked, September 2008
http://www.saps.org.uk/secondary/teaching-resources/293-student-sheet-24-microscale-investigations-with-catalase Microscale investigations with catalase – which has been transcribed onto this site at Investigating catalase activity in different plant tissues.
(Website accessed October 2011)

BIOLOGY TEACH
Enzyme activity: Definition, types and factors
Enzymes are essential biological catalysts that speed up chemical reactions in living organisms without being altered themselves. This article provides a thorough overview of enzyme activity, including the critical roles of enzymes, enzyme classification systems, kinetics, common assays, and the various factors influencing enzyme function.
What are Enzymes?
The critical roles of enzymes in cells and organisms, enzyme classification systems, enzyme kinetics, common enzyme activity assays, 1. temperature, 3. substrate concentration, 4. enzyme concentration, 5. inhibitors, 6. activators, lock and key model, induced fit model, cleft model, hand-glove model, references and sources.
Enzymes are primarily proteins that catalyze or increase the rate of biochemical reactions. The enzyme itself remains unchanged by the reaction and can be reused multiple times.
Without enzymes, many essential chemical transformations in cells would proceed extremely slowly or not at all.
Enzymes have an active site that provides a unique chemical environment to bind to specific substrates. This active site perfectly complements the substrate molecular structure and allows enzymatic catalysis to take place.
The substrate(s) binds to the active site through multiple weak interactions like hydrogen bonding, resulting in the enzyme-substrate complex.
In addition to proteins, a small subset of enzymes comprises catalytic RNA molecules called ribozymes. Examples include ribonuclease P and the hammerhead ribozyme. However, protein-based enzymes are far more abundant and diverse.
Enzymes carry out a vast array of essential functions in sustaining life. Some key examples include:
- Metabolic reactions: Enzymes catalyze all the complex sequences of metabolic pathways like glycolysis, Krebs cycle, electron transport chain, etc., that generate energy and synthesize important biomolecules.
- DNA replication: Helicases unwind the DNA double helix, while polymerases catalyze the synthesis of new complementary strands during replication.
- Gene expression: RNA polymerases synthesize RNA transcripts from DNA genes. Other enzymes process and splice RNA.
- Cell signaling: Kinases and phosphatases add or remove phosphate groups to regulate signaling proteins.
- Cell motility: Motor proteins like kinesin and dynein generate mechanical force through ATP hydrolysis to transport cargo within cells.
- Apoptosis: Caspases cleave regulatory and structural proteins as part of the programmed cell death pathway.
- Blood clotting: Thrombin, fibrin, and other enzymes trigger a cascade response to form blood clots upon injury.
- Digestion: Digestive enzymes like amylases, proteases, and lipases break down food polymers into absorbable molecules.
As evident, enzymes control practically every biological process and allow organisms to grow, reproduce, and sustain homeostasis. Defects in enzymes lead to metabolic disorders and diseases.
Enzymes are divided into different classes based on the type of reactions they catalyze. The International Union of Biochemistry (IUB) has developed a classification system that assigns each enzyme a 4-digit EC number.
The EC number indicates the enzyme class, subclass, sub-subclass, and serial number. For instance, lysozyme, which cleaves glycosidic bonds in bacterial cell walls, is designated as EC 3.2.1.17, where:
- 3 indicates it is a hydrolase (catalyzes hydrolysis reactions)
- 2 indicates it acts on glycosyl compounds
- 1 indicates it is a glycosidase
- 17 is the serial number of the specific enzyme in the sub-subclass
The 6 main IUB enzyme classes are:
- Oxidoreductases: Catalyze oxidation-reduction reactions involving electron or hydrogen transfer between molecules. Examples are dehydrogenases, peroxidases, and oxygenases.
- Transferases: Transfer functional groups like methyl, amino, and phosphate groups between substrates. Examples are kinases, transaminases, transmethylases.
- Hydrolases: Catalyze hydrolysis reactions that cleave bonds using water. Examples are proteases, lipases, phosphatases.
- Lyases: Catalyze non-hydrolytic addition or removal of groups from substrates. Examples are decarboxylases and dehydratases.
- Isomerases: Catalyze structural rearrangements within a single molecule. Examples are racemases and epimerases.
- Ligases: Catalyze bond formation between substrates by condensation reactions coupled to ATP hydrolysis. Examples are DNA ligase and acetyl CoA synthetase.
Alternatively, enzymes can also be classified based on the type of substrate they act upon like:
- Proteases act on protein substrates
- Lipases act on lipid substrates
- Polymerases act on DNA/RNA polymers
- Kinases act on proteins by phosphorylating them
Enzyme kinetics refers to measuring reaction rates and analyzing how kinetic parameters change with varying substrate/enzyme levels. This provides valuable insights into the catalytic mechanism and efficiency.
Key kinetic parameters are:
- Maximum velocity (Vmax): Maximum rate of the enzymatic reaction when the enzyme is saturated with substrate.
- Michaelis-Menten constant (Km): Substrate concentration at which the reaction rate is half Vmax. Indicates substrate-binding affinity.
- Turnover number (kcat): Maximum number of substrate molecules converted to product per enzyme active site per second. Indicates catalytic efficiency.
Enzyme kinetics is studied by measuring initial reaction rates at different substrate concentrations. A Michaelis-Menten kinetic plot depicts the hyperbolic relationship between reaction rate and substrate concentration.
Km and Vmax values are derived from such plots. Lower Km indicates higher substrate affinity, while higher Kcat indicates superior turnover capacity. High kcat/Km ratios signal enzymes with great catalytic power.
There are several assays to measure enzyme activity by monitoring reaction progress. Common methods include:
- Spectrophotometry: Measures absorbance changes as substrates are converted to products. Works for colored substrates/products.
- Fluorometry: Monitors fluorescence changes during reactions. Fluorescent substrates/products are used.
- Chromatography: Reaction components are physically separated and quantified. Works for non-colored substrates.
- Radiometry: Radiolabeled substrates are tracked throughout reactions. Sensitive, quantitative detection.
- Electrochemistry: Electron transfer during redox reactions is monitored using electrodes.
- Turbidometry: Measures changes in solution turbidity as insoluble products form.
- pH change: Hydrogen ion concentration changes can indicate reaction progress.
Assays should be designed to ensure initial rate conditions using low enzyme levels and excess substrate. Activity is calculated from the initial linear increase in product formation over time.
High-throughput screening uses automated assays to rapidly test enzyme variants or drug candidates that affect specific enzymes.
Factors Influencing Enzyme Activity
Many chemical and physical factors in the cellular milieu can affect enzyme activity and kinetics:
- Enzyme reaction rates increase with rising temperatures as kinetic energy and molecular collisions increase until an optimum temperature is reached.
- Each enzyme has an ideal temperature for maximum catalytic activity, generally between 35-45°C for human enzymes.
- At lower temperatures, enzyme activity is reduced due to decreased molecular motions and flexibility of the enzyme structure.
- However, above the optimum temperature, further heating begins to denature the 3D structure of the enzyme protein due to thermal instability.
- High temperatures break the weak bonds like hydrogen bonds and hydrophobic interactions that maintain folded conformation. This leads to unraveling and loss of active site geometry.
- Enzymes from thermophilic organisms have higher optimum temperatures due to specialized stabilizing structural features. But most enzymes become inactive by 70-80°C.
- Temperature not only affects the rate of enzymatic reactions but also the equilibrium position. The equilibrium constant of exothermic reactions decreases with rise in temperature.
- Each enzyme shows maximal catalytic activity at a specific pH known as the optimum pH. This is typically between pH 5-9 for most enzymes.
- Extremes of very high or low pH values usually lead to loss of proper enzyme structure and function due to denaturation.
- pH influences the ionization states of key amino acids involved in substrate binding and catalysis.
- Acidic or basic pH can protonate or deprotonate groups essential for activity. Electrostatic interactions are affected.
- Active site geometry depends on ion pairs formed between positively and negatively charged amino acids. Changing pH disrupts these critical ion pairs.
- Some enzymes, like pepsin, have evolved to function in highly acidic conditions, like a stomach pH of 1-3.
- Likewise, detergent enzymes work well in an alkaline pH of 8-10. But such extremes denature most other enzymes.
- The pH optimum varies based on where the enzyme normally functions. Cytoplasmic enzymes have optima near neutral pH, while digestive enzymes are optimized for acidic secretions.
- Buffers are used to maintain the pH close to the enzyme optimum both in vivo and in vitro experimental conditions.
- The reaction rate increases proportionally as the substrate concentration rises, providing more substrate molecules to bind to the active sites.
- However, at very high substrate concentrations, the enzyme active sites eventually get saturated as all sites are occupied.
- Beyond saturation, a further increase in substrate levels does not increase the rate since no new enzyme-substrate complexes can form.
- The maximum reaction rate or Vmax is achieved at substrate concentrations far above the enzyme’s Km value, indicating complete saturation.
- The substrate concentration for reaching Vmax depends on the enzyme-substrate binding affinity. Stronger binding enzymes require a lower substrate for saturation.
- Weak inhibitor compounds can also act as substrates at very high concentrations and elicit Vmax rates by overwhelming the inhibition.
- Substrate inhibition may occur at excessively high substrate concentrations due to multiple substrate molecules jamming active sites.
- The reaction rate is directly proportional to the enzyme concentration when other conditions, like substrate levels, are non-limiting.
- Having more enzyme molecules in the assay increases the total number of active sites available.
- Each active site can convert substrate to product at its kcat rate. So, higher enzyme levels linearly increase the overall reaction rate.
- This relationship helps determine enzyme levels needed for desired catalytic capacity based on substrate amounts.
- In cells, the total enzyme concentration is regulated at adequate levels to support flux through metabolic pathways.
- Enzyme production rates are controlled by gene expression regulation, translation, and protein degradation.
- Inhibitors are compounds that bind to enzymes and reduce their catalytic activity.
- Competitive inhibitors occupy the enzyme’s active site, competing with the substrate for binding. This obstructs substrate access to the site.
- Increasing substrate concentration can overcome this inhibition by out-competing the inhibitor.
- Non-competitive inhibitors bind at allosteric sites away from the active site. This changes enzyme shape and distorts the active site, reducing function.
- Uncompetitive inhibitors only bind to the enzyme-substrate complex, not the free enzyme. This also lowers activity.
- Irreversible or covalent inhibitors form permanent chemical bonds to active site residues. This irreversibly inactivates the enzyme.
- Most inhibitors display mixed inhibition, combining aspects of multiple modes. Inhibitors are used clinically to target key pathogens or disease enzymes.
- Enzyme activators are compounds that increase the catalytic activity of enzymes.
- Activators typically bind to specific allosteric sites on the enzyme, distinct from the active site.
- This induces a conformational change in enzyme shape that enhances its affinity for the substrate at the active site.
- The activator may also facilitate product release, allowing more rapid enzyme turnover.
- This modulation of activity provides a means of regulating metabolic flux through pathways in response to cellular needs.
- Enzyme cofactors like metal ions can act as activators by assisting in substrate binding and transition state stabilization.
- Oxidative enzymes generally require activating metal ions. The activator may also be a protein subunit in a multi-enzyme complex.
Models Explaining Enzyme Structure and Catalysis
Over the years, different models have been proposed to explain enzyme structure, substrate binding, and catalytic mechanism:
- First proposed by Emil Fischer in the 1890s based on shape complementarity.
- Envisioned the enzyme active site as having a rigid, predefined shape that fits only specific substrate molecules, akin to a lock and key.
- The binding of the substrate ‘key’ into the enzyme ‘lock’ occurs without any conformational changes.
- The lock and key analogy implies specificity but no induced fit. Only the correctly shaped substrate can bind to the active site.
- This model explains enzyme specificity well but does not account for kinetics or catalytic mechanisms beyond binding.
- Provides a simplistic preliminary explanation for selective substrate binding affinity seen in enzymes.
- Proposed by Daniel Koshland in 1958 to modify some limitations of the lock and key model.
- The active site is envisioned as being flexible and undergoing conformational changes upon substrate binding.
- The substrate interaction causes the active site to mold around it and induce fit for optimal binding.
- This adjusts the active site shape and charges to orient and stabilize the transition state for catalysis properly.
- The induced fit enhances substrate affinity and allows catalytic groups to interact with the bound substrate.
- Accounts for conformational dynamics and substrate-induced changes contributing to enzyme function.
- Suggests that the active site is a cleft or crevice rather than a pocket with defined boundaries.
- Substrate binding occurs through multiple weak interactions along the length of the cleft, not just within a pocket.
- It allows for some flexibility and movement, but the substrate itself does not change shape upon binding.
- Emphasizes distributed binding determinants rather than specific geometric complementarity seen in the lock and key model.
- An intermediate between very rigid and completely flexible interpretations of substrate binding.
- Conceptualized by Walter Kauzmann in 1959.
- An analogy of a hand fitting snugly into a glove; active site adjusts analogous to a glove around the substrate, which fits like a hand inserted into it.
- Combines aspects of both the rigid lock and key and flexible induced fit models.
- Some defined specificity exists, but the active site molds to the substrate like a glove.
- Accommodates subtleties of selective binding and optimized fit for transition state stabilization.
- No single model explains everything about enzyme kinetics and catalysis. The mechanism likely involves aspects of multiple models.
- Lehninger Principles of Biochemistry, 4th edition (David L. Nelson Michael M. Cox)
- 4.6 Enzymes – Human Biology. https://open.lib.umn.edu/humanbiology/chapter/4-6-enzymes/
- http://getfreeessays.com/effect-of-ph-and-temperature-enzyme-catalase-reaction-rate/
- Habte, Mezgebu Legesse, and Etsegenet Assefa Beyene. “Biological Application and Disease of Oxidoreductase Enzymes.” 2020, https://doi.org/10.5772/intechopen.93328.
- Green, Laura Kay. “In Vitro and in Vivo Characterisation of P. Aeruginosa Oxidoreductase Enzymes in Pathogenesis and Therapy.” 2012.
- Enzyme Inhibition. https://www.brainkart.com/article/Enzyme-Inhibition_27495/
- https://core.ac.uk/download/477734669.pdf.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Biology Teach
Biology teach is a platform, where you can find biology topics notes, lecture notes and video lectures, research papers, biology books, practical work, slides, and quizzes.
All Biology Notes
© 2019 - 2023 BIOLOGYTEACH All Rights Reserved
Privacy policy

IMAGES
VIDEO
COMMENTS
Data also showed that enzyme activity in the presence of added glucose solution was (barely) optimal at the 1/10 dilution factor. This directly conflicts with the conclusion that added glucose acts as an inhibitor as the 1/100 dilution factor should be the optimal concentration for enzyme activity in the presence of added glucose due to it ...
Factors affecting enzyme activity . Enzymes are sophisticated catalysts for biological processes. These practicals (and the practicals at intermediate level) give you opportunities to explore how enzyme activity changes in different conditions. Enzyme experiments often provide real 'messy' data, because their activity can change ...
EXPERIMENT NO. 6 Factors Affecting Enzyme Activity I. BACKGROUND. Enzymes are proteins that act as biological catalysts. They are highly efficient and increase the rate of the reaction by as much as 10 9 faster. Enzymes bind to their substrate, the substance that they act upon, via noncovalent interactions to form an enzyme-substrate complex.
Factors Affecting Enzyme Activity INTRODUCTION The chemical reactions occurring in living things are controlled by enzymes. An enzyme is a protein in the cell which lowers the activation energy of a catalyzed reaction, thus increasing the rate of the reaction. The enzyme which you will investigate in this exercise is peroxidase.
Propose a way to refine your experiment to find the exact, or OPTIMAL pH and temperature of catalase. 2. The following graph shows reaction rates of various enzymes in the body. Pepsin is found in the stomach, amylase in the saliva, and phosphatase in the liver. Synthesis: How does pH affect the activity of enzymes? Claim:
Experiment 10 - Enzymes Enzymes are proteins that act as catalysts for biological reactions. ... Constant temperature water baths will be needed for this experiment. The laboratory has a large water bath that can be set to 37°C, and everyone will use this water ... Make a graph of the enzyme activity vs. the amount of amylase solution using ...
LABORATORY REPORT Objective: Determine the cause and effect of substrate concentration on the initial rate of an enzyme-catalyzed reaction. Determine the effect of pH on the initial rate of an enzyme-catalyzed reaction. Introduction: This experiment is meant to show an understanding of how our body breaks down food. The highlight of this experiment is to show how the enzyme called amylase in ...
7 Lab 6. Enzymes - Catalase Function Lab 6: Enzymes - Catalase Function OBJECTIVES. Practice and apply hypothesis testing. Practice experimental design. Gain a better understanding of enzymes and some conditions that affect enzyme activity and the rate of an enzyme-catalyzed reaction.
Hydrogen peroxide is harmful and must be removed as soon as it is produced in the cell. Cells make the enzyme catalase to remove hydrogen peroxide. This investigation looks at the rate of oxygen production by the catalase in pureed potato as the concentration of hydrogen peroxide varies. The oxygen produced in 30 seconds is collected over water.
Factors Influencing Enzyme Activity. Many chemical and physical factors in the cellular milieu can affect enzyme activity and kinetics: 1. Temperature. Enzyme reaction rates increase with rising temperatures as kinetic energy and molecular collisions increase until an optimum temperature is reached.