- Earth Science
- Physics & Engineering
- Science Kits
- Microscopes
- Science Curriculum and Kits
- About Home Science Tools
Science Projects > Chemistry Projects > Acid Base Reactions & pH Experiments

Acid Base Reactions & pH Experiments
Experimenting with acids and bases can make for exciting chemistry projects!
Acidic solutions have a higher concentration of hydrogen ions (H+).
These are hydrogen atoms that have lost an electron and now have just a proton, giving them a positive electrical charge.
Basic solutions, on the other hand, contain hydroxide ions (OH-). One of the simplest activities to show how acids and bases react with each other (and to demonstrate their different properties) is to make a vinegar and baking soda volcano .
For another reaction experiment , put an Alka-Seltzer tablet in the bottom of a clear plastic film canister (the kind where the cap fits inside instead of closing over the outside).
Fill the canister with warm water and then quickly put the cap on and watch the acid-base reaction!

The pH scale is used to measure how acidic or basic a solution is. Acids have a pH below 7; bases have a pH above.
Neutral solutions (like distilled water) with a balanced number of H+ and OH- ions have a pH of 7. Do the following projects to explore the cool effects of pH.
Litmus is a natural acid-base indicator extracted from a type of lichen. If you have red and blue litmus paper , you can test different solutions for whether they are acids or bases.
Blue litmus paper turns red when a solution is acidic; red litmus paper turns blue in basic solutions.
Try testing window cleaner, toilet bowl cleaner, orange juice, and apple juice—pour a little of each into separate test tubes or small glasses or jars.
Use the litmus paper to determine which are acids and which are bases. Here are the pH levels of some other substances that you might test:
- Lemon juice (2)
- Vinegar (3)
- Egg whites (8)
- Baking soda (9)
- Ammonia (10)
Human blood has an ideal pH of 7.4; even slight fluctuations can seriously affect our bodies.
You can also make your own pH indicator —use a blender to mix one part chopped red cabbage with two parts boiling water and use the juice to test different solutions.
Acids will turn the pigments in the indicator to a reddish color; bases will turn the pigments bluish or yellow-green.
Mystery Pitcher
Make ordinary water turn bright pink and then back to clear! This makes a great “magic trick” to impress your friends – just be careful no one mistakes it for fruit punch and drinks any!
>> Check out our project video to see this trick in action!
What You Need:
- Phenolphthalein solution
- Sodium carbonate
- 5 glasses and a non-see-through pitcher of water
What You Do:
1. In the first glass put a little less than 1/8 teaspoon of sodium carbonate, in the second put 6 drops of phenolphthalein solution, and in the third put three droppers-full of vinegar.
2. Add a few drops of water to the first glass and stir to dissolve the sodium carbonate.
3. Fill all the glasses with water from the pitcher, then pour all of them back in the pitcher except for the glass with vinegar.
4. Refill the remaining four glasses – the water will be red!
5. Now pour all five glasses back in the pitcher. Refill the glasses one last time—the liquid will be colorless again!
What Happened:
Phenolphthalein is a pH indicator, but it only turns colors in reaction to bases. When you poured the four glasses back into the pitcher, the phenolphthalein reacted to the sodium carbonate, a base, and turned the solution to bright pink “kool-aid.” To change it back to “water,” all you had to do was add the acidic vinegar, which turned the phenolphthalein colorless again.
Rainbow Reaction Tube
Amaze your friends by mixing two solutions to make a rainbow!
Watch as purple sinks to the bottom and red floats to the top, and they mix together to form every color in between.
- 10ml graduated cylinder
- Universal indicator
- Distilled white vinegar

2. Add 3 drops of vinegar to the solution in the graduated cylinder, and it should turn red.
3. In a beaker, put two scoops of sodium carbonate and then add about 30 ml of water. Mix together with the stirring rod until the sodium carbonate dissolves. The solution should be clear.
4. To start the reaction, fill one dropper full with sodium carbonate solution. Squeeze the dropper into the graduated cylinder quickly, rather than drop by drop. The clear solution should instantly turn dark purple, and slowly sink to the bottom, swirling around to make the rainbow.
5. Let the contents of the cylinder settle, until you can see each color from bluish-purple to red. To make the rainbow disappear, pour it into an empty beaker, and it should turn yellow or yellowish green.
Universal indicator changes colors to show the pH level of a substance. In this case, when you mixed an acidic solution (vinegar) with a basic one (sodium carbonate), the indicator made a colorful spectrum — from dark blue to red. Interestingly, if you had added the solutions in the opposite order, you would not have seen a rainbow. To get the rainbow effect, another scientific principle is at work— density . The sodium carbonate solution you made is denser than the indicator solution, so it sinks to the bottom. As the sodium carbonate solution makes its way to the bottom, some of its molecules mix with vinegar molecules, making a new solution, which shows up as a color of the pH scale.
If you don’t turn the graduated cylinder upside down, the rainbow will last several days. Over time the colors will mix together through the process of diffusion. The molecules of each solution will mix throughout the graduated cylinder, rather than staying concentrated at the top or bottom. Once you mix the acid and base solutions together, the solution will be pH neutral, and look yellow or slightly green.
To make a different kind of rainbow tube, try making this rainbow density column with all household materials.
More Chemistry pH Projects:
- Green Eggs & Ham
- Fizzy Bath Bombs
- Acid & Apples
- Copper-Plated Nails
Welcome! Read other Chemistry articles or explore the rest of the Resource Center, which consists of hundreds of free science articles!
Shop for Chemistry Supplies!
Home Science tools offers a wide variety of Chemistry products and kits. Find affordable beakers, test tubes, chemicals, kits, and everything else you need for lab experiments.
Related Articles

Science Project Name Suggestions
Science Project Name Suggestions Thinking of a name for science fair projects for 7th graders or science fair projects for 8th graders is all part of the fun. Your kid’s sense of creativity will be challenged, and the unique name they come up with can boost their...

Science Fair Projects for 8th Graders
Science Fair Projects for 8th Graders As kids reach the 8th grade, their exposure to science goes up a notch. Equipped with basic knowledge, they can begin to explore more complicated concepts and satisfy their curiosity for deeper answers to the 'whys' and 'hows' of...

Science Fair Projects for 7th Graders
Science Fair Projects for 7th Graders Science fair projects for 7th graders are a step up in complexity. Because 7th graders have a better grasp of science concepts, they’re expected to practice the scientific method in the way they approach their experiments–which...

Home Science Experiments for Preschoolers
Home Science Experiments for Preschoolers Home science experiments for preschoolers are a great way to pique your child’s curiosity, teach them valuable knowledge, and allow them to have some fun in the comfort of their own home. There are plenty of activities your...

Easy Science Fair Projects for Kids
Easy Science Fair Projects for Kids Science fairs are a long-standing tradition that provide kids with the opportunity to better understand practical concepts in fun and innovative ways. The great thing about the experiments presented at these events is that they...
JOIN OUR COMMUNITY
Get project ideas and special offers delivered to your inbox.
Science Experiments On pH Levels

Testing the pH level of a substance tells you if that substance is acidic, basic or neutral. The pH scale ranges from 1 to 14; 7 is neutral, lower numbers are acidic, and higher numbers are basic. Science experiments on pH levels help investigators determine the pH level of a given material and how that level might affect the environment. These experiments can illustrate important processes such as the effect of acid rain on bodies of water.
Comparing Everyday Products
One simple experiment to get started exploring pH levels involves testing several everyday items you might use at work or home. Collect items such as cleaning supplies, soda pop, water, milk, vinegar, laundry detergent, lemon juice, shampoo, mouthwash or even your own saliva or sweat. Label glass jars or cups with a wax pencil for each liquid you test. Fill the jar 1/3 to 1/2 full with liquid, except saliva or sweat, which can be collected on a cotton swab. Place the tip of a piece of litmus paper or pH indicator paper into the liquid for two seconds; remove the paper and record the color you see. Acidic substances will show a yellow or red color, while basic substances will show blue. Neutral substances appear green. Record and compare your results — were your chosen substances acidic or basic? Did they differ from what you expected?
Comparing Water Samples
Using similar techniques, you can test the water supply in your area to determine whether or not it is neutral. Most water supplies have a pH between 6 and 8, but due to acid rain, some bodies of water have lower pH levels, meaning they are more acidic. Collect water from various water sources around your home or collect rainfall in a clean glass jar and secure it with a lid. You can even make a map of where the water samples were gathered. Test each water sample with litmus or pH paper and record its color. Does one area around your home have more acidic pH? How acidic is the rainfall in your area?
The Effects of pH on the Teeth
Many types of soda pop contain acids to enhance taste, and these can have a corrosive effect on human teeth. To study these effects, fill containers with several brands of soda, being careful to put just one type of soda in each container. As controls, fill one more cup with clear water and one with vinegar, which is highly acidic.
Measure the pH of the contents of each container with litmus paper and record the results. Place a piece of eggshell in each container. Eggshells are made of mostly the same compounds of human teeth. Notice what happens to the eggshells and record the results. You'll probably notice that deterioration increases with increasing pH, that the eggshells in water suffer no effects. To eliminate any possible effects of carbonation, allow the containers containing soda to sit uncovered for several hours before you add the eggshells.
Soil Buffering
Some soils have substances that buffer — or act to neutralize — acids or bases. You can put soil from your own backyard to the test. Collect enough soil to fill a coffee filter. Put the coffee filter in a funnel and put the soil into the filter, but do not pack down the soil. Create an acidic mixture of 2 tablespoons vinegar and 2 cups distilled water. Test the acidity with pH test paper; add water or vinegar until the mixture has a pH of around 4. Hold the funnel over a paper cup and pour the water over the soil. Check the pH of the water that collects in the paper cup. If the pH remains the same, the soil did not buffer the acid, but if the pH level rises, the soil did buffer the acid.
- Environmental Protection Agency: Science Experiments
- Minnesota Science Teachers: pH of Household Items
- Canadian Dental Association: EggSperiment Activity
Cite This Article
Batema, Cara. "Science Experiments On pH Levels" sciencing.com , https://www.sciencing.com/science-experiments-ph-levels-9174/. 27 April 2018.
Batema, Cara. (2018, April 27). Science Experiments On pH Levels. sciencing.com . Retrieved from https://www.sciencing.com/science-experiments-ph-levels-9174/
Batema, Cara. Science Experiments On pH Levels last modified August 30, 2022. https://www.sciencing.com/science-experiments-ph-levels-9174/
Recommended

Acid and Base Experiment
Sharing is caring!

When studying chemicals, one of the main characteristics scientists look at is the pH level. This determines whether the chemical is an acid, a base, or neutral. Using this knowledge, scientist can predict how the chemical will react with other substances. Use this pH lesson to learn more.

What is pH? What is an Acid and Base Experiment?
What is ph.
The pH level of a chemical refers to the concentration of hydrogen ions in a solution. What is meant by that?
When molecules are put into water, a solution is created. Sometimes these molecules break down in the solution and release a hydrogen (H+) ion. At other times, the molecules will release a hydroxide (OH-) ion. When a hydrogen ion is released, the solution becomes acidic. When a hydroxide ion is released, the solution becomes basic, or alkaline.

Learn About The pH Scale
The acidity or alkalinity of a solution is measured using the pH scale. This pH scale is used by scientists to measure how acidic or basic a liquid is on a scale from 0 to 14. The lower the pH number on the scale the more acidic a liquid is and the higher the number the more basic. Liquids with a pH of 7 are considered neutral.
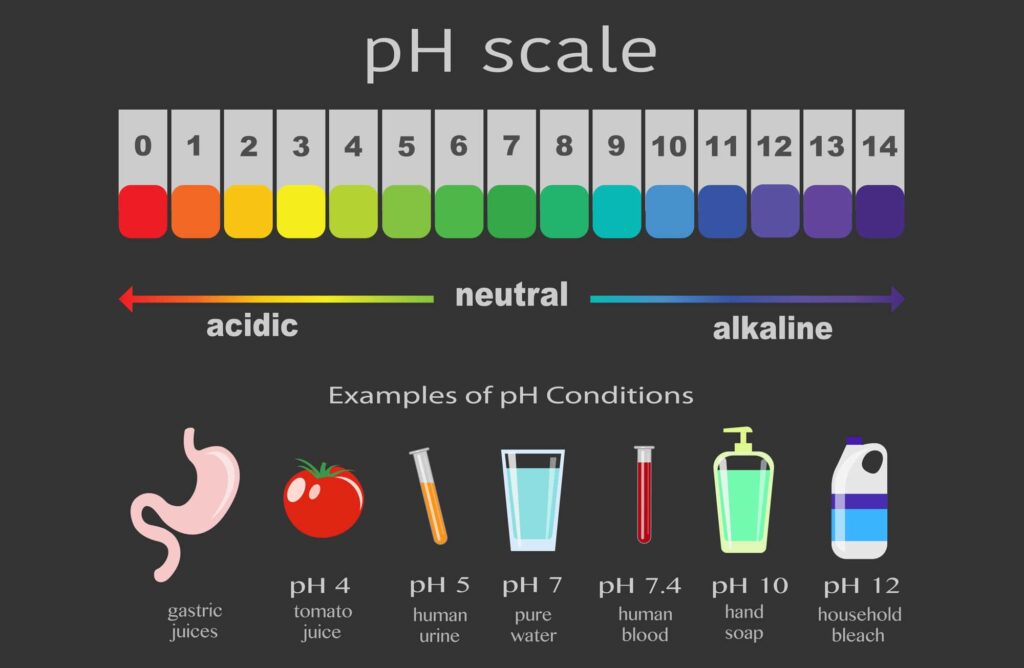
The more acidic or basic a solution is the more reactive that solution is with other substances. This means more chemical reactions take place. This is because of the large amounts of hydrogen and hydroxide ions available to react.
Strong acids, such as battery acid, and strong bases, such as sodium hydroxide, are highly reactive and dangerous to handle. These are very corrosive and should be handled in a laboratory or industrial application where great protective measures are taken.
Weak acids, such as acetic acid, and weak bases, such as ammonia, are not as reactive because of the lower amounts of hydrogen and hydroxide ions available, respectively. Even though these are considered acids or bases, they are relatively safe to handle.
How To Measure pH
We have already learned that scientist can use the pH scale to determine the acidity or alkalinity of a solution. But, how do we measure the pH of solutions?
One method to determine pH is to use indicator solutions. These are chemicals that when added to a solution change the color of the solution based upon the pH of that solution. Common indicators are phenolphthalein, methyl orange, methyl red, bromothymol blue, and thymol blue. They each change color at different points on the pH scale. These indicators are all found in one universal indicator solution that can determine the pH level across the entire scale or targeted to a smaller range of the scale. The more targeted, the more accurate the result will be.
Universal indicator solutions can be used in their liquid form and dropped into solutions or universal indicator solutions can be used to treat paper to create pH strips . These strips can be dipped into liquids and the strips will change color according to the pH of the liquid. Comparing the colors to a chart will help determine the pH of the liquid.
Here is a great video about using universal indicator solution by dropping it into a liquid –> https://www.youtube.com/watch?v=GRNslAaXu9k
For a great pH paper experiment, check out the end of this post!
Another method to determine whether a solution is an acid or base is to use litmus pape r. These paper strips are treated with special indicator chemicals, but can only tell you if a solution is an acid or base. Blue litmus paper will turn red if the solution is an acid and red litmus paper will turn blue if the solution is a base. Litmus paper does not tell you the strength of the acid or base. You can make your own litmus solution with cabbage. Learn how in this post.

Download the Acid & Base Experiment Worksheets
Setting up an acid and base experiment.
Every liquid in your home is either an acid, a base, or neutral. An easy way to find out the pH of liquids you handle everyday is by using pH strips. . Dip the strips into various liquids to see for yourself.

The other day, we were reading an article from LaTrobe University about how brewing a great cup of coffee depends on chemistry and physics . According to the article, one variable that affects the taste of a cup of coffee is the chemistry of the brew water. It was also interesting to read about the physics of the coffee particles!
But, what about the pH level of other substances we use in cooking or might use in cleaning?
This got us testing the pH levels of pantry items such as green tea, vinegar, spaghetti sauce, and more.
Plus, we tested some household cleaning items, like oven cleaner and shower cleaner. (These are highly toxic, so I recommend they be handled wearing gloves and a mask, and sprayed outdoors into a little bowl.) I wanted to use the cleaning items because I knew they would give us a very alkaline (basic) reading.

Design your own pH experiment using other common household liquids and record your results. You might want to try this with orange juice, liquid bleach, different kinds of soft drinks, milk, apple juice, glass cleaner, detergent, and saliva. Before you test them. make some predictions about whether each is an acid, base, or neutral.
Use this pH lesson and pH experiment with your students. Let me know what liquids you tested and if you had any surprises.
- 1 package pH testing paper
- 8 to 12 bowls or containers
- Substances to test – orange juice, lemon juice, tea, coffee, sauces, household cleaners
- 1 pair lab glasses
- 1 pair disposable gloves
- 1 face mask, if testing aerosol cleaners
Instructions


For more chemistry experiments, lessons and resources…

I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.

Acids and Bases Experiment Exploring pH Levels
Using items from the kitchen this fascinating experiment explores Acids and Bases and pH Levels. Kids will love digging through the pantry to test out whether items are an acid or a base, and explore pH levels of every day items. An excellent elementary chemistry experiment for hands-on with science with lots of further studies.
Acids and Bases Chemistry Experiment Exploring pH Levels
Table of Contents

My kids LOVE cabbage. I know, that isn’t something you hear every day, but they do! So when I mentioned one day that we could use their beloved cabbage for science, my kids were instantly curious. The idea that one of their favourite foods could do really cool things, other than taste good, was exciting.
This experiment is best done in the kitchen, but could be easily adapted for classroom use too. It takes about 30 to 45 minutes to complete and can be easily done as a demonstration with a group.
Acids and Bases Experiment Supplies
Red Cabbage (it’s actually purple… just in case you were confused) Knife Cutting board Hot water Blender Fine Mesh Strainer Large container (I used a 2 cup Pyrex glass measuring cup) Test tubes (because it’s cool, but small glasses would work too) Pipettes Variety of items to test
Preparing pH Indicator
Coarsely chop the red cabbage. I found 2 good sized slices was enough.
Add cabbage to the blender. Add hot water, the amount is pretty flexible. Just aim for about how much pH solution you need.
You can see how the water immediately starts to turn a beautiful blue colour.

Pour the blended mixture through the fine mesh strainer into a container like pyrex so it is easy to pour. Let cool.
If it is too dark, feel free to add some water to dilute it and lighten the colour.
The pH indicator water can be prepared ahead of time for classroom use. We saved ours for a few days with no problems.
Testing Acids and Bases
The next step is the coolest part, testing items to determine their pH. For this I let the kids go crazy picking items from the kitchen.
- Lemon juice
- Baking Soda
- Baking Powder (Big surprise here!)
- Corn Starch (Oops! Guess what this made?)
- Castille Soap
- Laundry Soap
- Rubbing alcohol
For our experiment we set out all the items we wanted to test. We used pipettes for the liquids and a small spoon for the powders. Make sure you clean your pipettes if you need to reuse them. It is best if you can have one pipette for each liquid.
After filling our test tubes with pH indicator we started adding our items. For anything we were not 100% sure about or that were a chemical, I did the testing for safety reasons.
And the results were amazing!!!
Acids, Bases and pH Levels Experiment
The pH solution starts out purple at a neutral pH of 7. Once we started adding other items, the colours started changing immediately.
Acids changed to a variety of pinks and reds.

While bases turned our indicator to a variety of greens, blues and even yellow!

There were some surprises.
First, adding corn starch to our pH indicator solution created… yup… oobleck . But that lead to the idea to try another experiment – Color Changing Oobleck that even gave us some more scientific surprises.
Second, some of our test tubes didn’t mix, so we ended up with a blend of colours where the liquids with various densities floated on top of one another.
Third, and this one surprised us all… did you know Baking Powder erupts, releasing CO2 gas bubbles when mixed with water? We didn’t, so that was a BIG surprise! Turns out Baking Powder is both an acid and a base, but it requires water be added before the acids and bases react to produce CO2 gas. We ended up doing a follow up scientific investigation into Baking Powder vs Baking Soda here .
See, you never know what you might learn in an experiment! In fact this discovery was so much fun for my kids that they set up a Baking Powder experiment where they tested adding it to a variety of liquids to see how it changed the reactions.
I swear, sometimes it’s like science multiples on itself around here.
Ph indicator rainbow .
Speaking of new experiments, we decided to try making our own pH rainbow and layer the reaction by adding a base at the bottom of a large test tube filled with pH indicator, and acid to the top. The result was kind of cool and swirly.

This experiment was a HUGE success and led to so many questions and new experiments and studies. Plus we used items from our kitchen. And Kitchen Science is always awesome!
Try more fun with pH indicators in this Invisible Ink Experiment .

5 Days of Smart STEM Ideas for Kids
Get started in STEM with easy, engaging activities.
All Science Fair Projects
1000 science fair projects with complete instructions.

25 Acid and Base Science Projects & Intro to Acids and Bases
Introduction to acids and bases, strong and weak acids and bases.
In acid-base chemistry, we encounter both strong and weak acids and bases.
Hydrochloric acid and sulfuric acid are examples of strong acids. These acids dissociate completely in water, releasing a high concentration of hydrogen ions. On the other hand, acetic acid (vinegar) and citric acid (found in citrus fruits) are weak acids. They only partially dissociate, resulting in a lower concentration of hydrogen ions.
Sodium hydroxide is a strong base, readily dissociating in water and releasing a high concentration of hydroxide ions (OH-). In contrast, a weak base like ammonia has a lower concentration of hydroxide ions.
What is pH? pH is an abbreviation for " p otential of H ydrogen," and it's a measure of the acidity or alkalinity of a solution.
The pH scale represents a solution's concentration of hydrogen ions (H+), with lower numbers indicating higher acidity and higher numbers signifying higher alkalinity. The scale ranges from 0 to 14, where 0 represents the highest acidity, 7 is neutral, and 14 indicates the highest alkalinity. Acids have a pH below 7, with strong acids typically falling between 0 and 3. Conversely, bases have a pH above 7, and strong bases typically range from 10 to 14.
Measuring pH
The most common way for measuring pH is using pH strips. The solution to be tested is dripped onto the pH strip or the strip is dipped into the solution, causing a color change to the strip. To find out the pH value, the resulting color is matched against a color chart that displays the corresponding pH range.
For more precise and accurate pH measurement, pH meters are utilized. These meters are calibrated using buffers of known pH and are immersed into the solution being tested. The pH value is conveniently displayed on a digital meter, ensuring a reliable reading.
Neutralization Acid-Base Reactions
When an acid and a base react, they undergo a process called acid-base neutralization. During this reaction, the acid donates a proton (H+) to the base, forming water (H2O) and a salt as products. This reaction balances acidity and basicity, achieving a state of equilibrium.
For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), they combine to form water (H2O) and sodium chloride (NaCl). HCl + NaOH → H2O + NaCl
A common neutralization reaction is that between vinegar (acetic acid) and baking soda (sodium bicarbonate), which forms carbon dioxide gas, water and sodium acetate. NaHCO3 + HC2H3O2 → CO2 + H2O + NaC2H3O2
Buffer Acid-Base Reactions
A buffer solution plays a crucial role in maintaining pH stability by resisting big changes towards becoming acidic or basic. It is composed of a combination of a weak acid and its conjugate base or a weak base and its conjugate acid. This specific combination allows the buffer to effectively absorb or release hydrogen ions (H+) in response to what's added to the solution. When an acid or base is added to the buffer, the weak acid-base pair present in the buffer system reacts to neutralize the added ions, preventing drastic shifts in pH. By providing a constant pH, the buffer solution is an essential component in various chemical reactions and biological processes.
The Arrhenius Theory of Acids and Bases in Aqueous Solutions
The Arrhenius theory, proposed by Svante Arrhenius, provides a foundational understanding of acids and bases. According to this theory, acids are substances that release hydrogen ions (H+) when dissolved in water, while bases release hydroxide ions (OH-). Acids increase the concentration of hydrogen ions, resulting in higher acidity, while bases increase the concentration of hydroxide ions, leading to alkalinity. The Arrhenius theory helps us understand acid-base reactions in aqueous solution.
The Brønsted-Lowry Theory of Acids and Bases and Proton Transfer
The Brønsted-Lowry theory, formulated by Johannes Nicolaus Brønsted and Thomas Martin Lowry, expands the definition of acids and bases beyond aqueous solutions. In this theory, an acid is a substance that donates a proton (H+), while a base is a substance that accepts a proton. The focus shifts from the dissociation of ions to proton transfer. When an acid donates a proton to a base, it forms a conjugate base, while the base becomes a conjugate acid.
The Lewis Theory of Acids and Bases and Electron Pair Donation
The Lewis theory of acids and bases, proposed by Gilbert N. Lewis, focuses on electron pair donation. According to this theory, acids are substances that accept electron pairs, while bases are substances that donate electron pairs. This theory extends our understanding of acid-base reactions beyond proton transfer and includes reactions involving non-aqueous solutions and gases.
Acids and bases play essential roles in countless chemical reactions and natural phenomena. From the sour taste of citrus fruits to the digestion of food in our stomachs, acid-base chemistry surrounds us. After understanding the properties, behavior, and theories of acids and bases, try your hand at acid-base chemical reaction science projects!
Browse Acid and Bases Science Fair Projects
Learn more about acids and bases with these science projects that have complete instructions based on the scientific method with accompanying videos!
More related science projects

Play of the Wild
Education is an admirable thing, but it is well to remember from time to time that nothing that is worth knowing can be taught. -Oscar Wilde

Turmeric pH Indicator Experiment for Acids & Bases

Turmeric pH Indicator Experiment for Acids & Bases
This turmeric pH indicator experiment for acids and bases is a fun, easy way to learn about acids and bases. If done with gentle household items like vinegar, lemon juice, baking soda, etc. it is safe even for young children.
Turmeric is a natural pH indicator. It changes colour in the presence of acids and bases. In the presence of acids, turmeric remains yellow, but in the presence of alkaline substances (bases), it turns red. It is ideal to use it for doing experiments with vinegar or citric acid and baking soda.

pH and chemical reaction – A simple summary
The pH is the measure of how acidic or basic (alkaline) a substance is. It is represented in a numerical range from 0 to 14, with seven being neutral. The more acidic the material, the closer it is to 0, the more basic, the closer it is to 14. The pH number indicates the presence or absence of hydrogen ions. Acids have hydrogen ions (H+), and bases have hydroxide ions (OH-). The higher the concentration of these ions, the more acidic or basic the substance.
When an acid and base come in contact with one another, a chemical reaction occurs. Acids and bases neutralize one another by breaking apart and forming into new substances. The reaction always results in the creation of water (H2O) and a salt.

If you want some more information about pH and colour changing experiments, you may want to see my Color pH Baking Soda and Vinegar Science Experiment .
What you need for a turmeric pH indicator experiment for acids and bases
- Baking soda/bicarbonate of soda
- Vinegar, lemon juice or citric acid
- Ice cube tray (optional)
There are different ways to do this experiment to make it exciting and fun for children.
The simplest way is for children to add different substances to some turmeric to see what happens. They could test substances such as lemon juice, soda, baking soda, water, etc. to see which one cause turmeric to change colour.
Once they have explored using turmeric as an indicator, they can have some fun with it. One option is to take some baking soda and mix in some turmeric. I usually use 2-3 teaspoons of turmeric for every ½ cup of baking soda. You will also need to add in a few teaspoons of water to help mix it all together. Once it is well combined, spread it out on a tray. Children can then squirt lemon juice or vinegar onto it using a pipette to see what happens.

Alternatively, you can also try freezing the baking soda.
To do this, prepare the turmeric, baking soda and water mixture as above. However, you may need to add a little more water to this mixture so that it will stick together. Scoop it into ice cube trays or moulds and place it in the freezer for several hours.

Once it is frozen, children can place the frozen cubes in a plate or bowl full of vinegar or citric acid. Alternatively, they can squirt the cubes with vinegar to create a reaction.
Questions to ask
- What do you notice about the reaction?
- What happens to the turmeric when it comes into contact with different substances?
- Is there a difference when you freeze the bicarbonate of soda rather than leave it room temperature?
- What do you notice when you combine the baking soda and vinegar when the turmeric is present?
- Could you think of a way to prove that a new substance is created after the reaction? What could you do?
- What happened? Why do you think it happened? What did you learn?
- Is there anything more you would like to try out or test?
What they get from it
This experiment will help children to explore the changes in pH that occur as part of a chemical reaction. They may also begin to think about whether there might be other ways to demonstrate that new and different substances form as part of a chemical reaction. This hands-on activity will teach them to predict, observe, record and discuss, or present their findings. Doing experiments will provide children with an opportunity to write by recording their observations with notes or drawings. Children enjoy doing experiments, and it can be highly motivational to write, even for more reluctant writers.
Research shows that people learn best (for long-term memory at least) when they learn through hands-on, practical experiences. (Hearns, Miller & Nelson, 2009; Hillman, 2011; Ferri, B.H., Ferri, A.A., Majerich, D.M., Madden, A.G., 2016). It’s also an opportunity for children to begin to develop scientific thinking. Furthermore, inquiry-based learning will encourage children’s curiosity and love of learning. (Ambrose et al. 2010; Froyd 2008; Prince & Felder, 2007; Springer, Stanne & Donovan, 1999).

Take it further
See my other vinegar and baking soda experiments so that children can explore chemical reactions:
- Baking Soda and Vinegar Painting Experiment for Kids (coming soon)
- Color pH Baking Soda and Vinegar Science Experiment
- Colorful Baking Soda and Vinegar Experiment for Kids
- Frozen Bicarbonate of Soda and Vinegar Experiment
- Valentines STEM Art Projects for Toddlers and Kids .
- You may also want to see my post on pH (acid-base) indicators ( coming soon ).
It can be great for children to create their own experiments based on what they want to find out. Allow them to ask questions to extend their learning. Ask them: What questions do you have about these reactions? Can you design an experiment to answer one of the questions?
They may want to see if they can find the exact amount of vinegar to make the baking soda react. They might want to see if a frozen baking soda ball reacts faster with vinegar than a dried baking soda ball. Alternatively, they may want to see if there is a difference in the reaction between vinegar and citric acid.
I hope that you enjoy trying out using turmeric as a pH Indicator in an experiment with acids & bases.
Ambrose, S. A., Bridges, M. W., DiPietro, M., Lovett, M. C., & Norman, M. K. (2010). How learning works: Seven research-based principles for smart teaching . San Francisco, CA: Jossey-Bass. https://books.google.com/books
Ferri, B.H., Ferri, A.A., Majerich, D.M., Madden, A.G. (2016). Effects of In-Class Hands-On Laboratories in a Large Enrollment, Multiple Section Blended Linear Circuits Course. Advances in Engineering Education, 5 (3). https://files.eric.ed.gov/fulltext/EJ1121997.pdf
Froyd, J. E. (2008). White paper on promising practices in undergraduate STEM education . Commissioned paper, Board on Science Education, National Academies. Retrieved from https://sites.nationalacademies.org/DBASSE/BOSE/DBASSE_080106#.UUoV5hngJ8g
Hearns, M.K., Miller, B.K. and Nelson, D.L. (2009). Hands-On Learning versus Learning by Demonstration at Three Recall Points in University Students. OTJR: Occupation, Participation and Health, 30 (4), 169-171. https://journals.sagepub.com/doi/10.3928/15394492-20090825-01
Hillman, C.N. (2011). The effects of hands-on learning versus learning by demonstration on memory in community-dwelling older adults (Doctoral dissertation, The University of Toledo). Retrieved from https://pdfs.semanticscholar.org/6231/d55fc1c730ec086f012677c54141f466e18e.pdf
Prince, M., & Felder, R. (2007). The many facets of inductive teaching and learning. Journal of College Science Teaching, 36 (5), 14–20. https://s3.amazonaws.com/academia.edu.documents
Springer, L., Stanne, M. E., & Donovan, S. (1999). Measuring the success of small-group learning in college-level SMET teaching: A meta-analysis. Review of Educational Research, 69 , 21–51. http://archive.wceruw.org/cl1/CL/resource/scismet.pdf
Physics & Chemistry , Preschooler , School Age
Baking soda , bicarbonate of soda , chemical reaction , colour , learning outside , pH , science experiment , Vinegar
2 thoughts on “ Turmeric pH Indicator Experiment for Acids & Bases ” Leave a comment ›
- Pingback: Sun Art for Kids and Toddlers – Play of the Wild
- Pingback: Acid and Base Experiment-Turmeric pH Indicator – Play of the Wild
Leave a Reply Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

IMAGES
COMMENTS
The pH scale is used to measure how acidic or basic a solution is. Acids have a pH below 7; bases have a pH above. Neutral solutions (like distilled water) with a balanced number of H+ and OH- ions have a pH of 7. Do the following projects to explore the cool effects of pH. Litmus is a natural acid-base indicator extracted from a type of lichen.
Apr 27, 2018 · The pH scale ranges from 1 to 14; 7 is neutral, lower numbers are acidic, and higher numbers are basic. Science experiments on pH levels help investigators determine the pH level of a given material and how that level might affect the environment. These experiments can illustrate important processes such as the effect of acid rain on bodies of ...
Learn About The pH Scale. The acidity or alkalinity of a solution is measured using the pH scale. This pH scale is used by scientists to measure how acidic or basic a liquid is on a scale from 0 to 14. The lower the pH number on the scale the more acidic a liquid is and the higher the number the more basic. Liquids with a pH of 7 are considered ...
This is a simple "kitchen chemistry" project about acid/base chemistry. Scientists measure the acidity or alkalinity of a solution using a logarithmic scale called the pH scale. In this project you'll learn about the pH scale, and you'll make your own pH indicator paper using a pH-sensitive dye that you'll extract from red cabbage.
Mar 5, 2021 · Acid and Base Experiment- What is Ph for Kids. In this post, we will look at how to do an acid and base experiment to help kids understand what pH is and how it is measured. What is pH, Acids and Bases (Explanation for Older Children) The pH measures how acidic or basic (alkaline) a substance is. It ranges from 0 to 14, with 7 being neutral.
Nov 20, 2023 · Using items from the kitchen this fascinating experiment explores Acids and Bases and pH Levels. Kids will love digging through the pantry to test out whether items are an acid or a base, and explore pH levels of every day items. An excellent elementary chemistry experiment for hands-on with science with lots of further studies.
Measuring pH. The most common way for measuring pH is using pH strips. The solution to be tested is dripped onto the pH strip or the strip is dipped into the solution, causing a color change to the strip. To find out the pH value, the resulting color is matched against a color chart that displays the corresponding pH range.
May 10, 2020 · Turmeric pH Indicator Experiment for Acids & Bases. This turmeric pH indicator experiment for acids and bases is a fun, easy way to learn about acids and bases. If done with gentle household items like vinegar, lemon juice, baking soda, etc. it is safe even for young children. Turmeric is a natural pH indicator.
Here at Precision Laboratories, we’re all about chemistry, and you might have noticed from our wide range of pH test strips that we’re obsessed with pH. From our most basic litmus papers to our narrow-range plastic pH test strips, we’ve got you covered. But sometimes, it’s just more fun to see some exciting acid base
Oct 12, 2023 · This science experiment uses common kitchen ingredients to learn about the pH scale which is great for homeschool, home fun or in the classroom. Let’s make our own tie dye and explore acid & bases! The other awesome thing about this easy chemistry lesson is that it kept the attention of kids from 3 year olds to 14 year olds!