Boyles law possible sources of errors?
Boyle's Law relates gas pressure (P) to volume (V) by the equation P1V1 = P2V2. There are two sources of errors to experimentally and theoretically applying Boyle. The first is based on instrumentation, which include operator errors and certainty problems when measuring P and V. The second is from calculations, specifically user errors.
Possible sources of errors in Boyle's law experiments include temperature changes affecting gas volume measurements, leaks in the apparatus altering pressure readings, and incomplete removal of air from the system leading to inaccurate results. Additionally, human errors in recording data and improper calibration of equipment can also contribute to deviations from the expected outcomes.
Botho Maitumelo Joha... ∙
Is it possible to use nitrogen in the experiment of Boyles law pressure and volume

Add your answer:
Which pioneer was best known for the discovery of a law that helps to explain characteristics of gases?
Robert Boyle is best known for Boyle's Law, which describes the relationship between pressure and volume of a gas at constant temperature. This law helps to explain the behavior of gases and is a significant contribution to the field of gas chemistry.
What gas law is P1v1p2v2?
This equation represents Boyle's Law, which states that the initial pressure multiplied by the initial volume is equal to the final pressure multiplied by the final volume for a given quantity of gas at constant temperature.
The variable that Boyles law holds constant is?
The variable that Boyle's law holds constant is the temperature. Boyle's law states that the pressure of a gas is inversely proportional to its volume, as long as the temperature remains constant.
What is the equation for boyles law?
Boyle's Law states that the pressure of a gas is inversely proportional to its volume when temperature is kept constant. Mathematically, it can be expressed as (P_1 \times V_1 = P_2 \times V_2), where (P) represents pressure and (V) represents volume.
When the properties of gas does not apply the boyles law?
Boyle's Law applies to ideal gases under constant temperature conditions. It does not apply to real gases or when extreme pressures or temperatures are present, as these conditions can cause gas molecules to deviate from ideal behavior. It is important to consider the limitations of Boyle's Law when dealing with non-ideal gas behavior.
What are some real life applications of Boyle's law?
When you pop a balloon by overfilling it with air, you are applying Boyles Law. When a nurse fills a syringe before she gives you a shot, she is working with Boyles Law. Sport and commercial diving. Underwater salvage operations rely on Boyles Law to calculate weights from bottom to surface. When your ears pop on a plane as it rises from takeoff, that's Boyles Law in action.
How are boyles law and Charles law similar?
They are both gas laws?
What is Boyles law well known for?
Boyle's Law is the inverse relationship between pressure and volume.
Pressure and volume change at a constant temperature who's law is this?
What are the possible sources of error or limitation in boyles law experiment.
Possible sources of error in a Boyle's Law experiment include air leaks in the apparatus, temperature fluctuations affecting the volume of the gas, incorrect readings due to parallax error, and deviations from ideal gas behavior at high pressures. Limitations include the assumption of ideal gas behavior, which may not hold true at all conditions, and the difficulty in accurately measuring the volume of the gas at high pressures.
Is boyles law a direct or indirect relationship?
Boyle's Law is an indirect relationship. (Or an inverse)
How are Boyles law and Charles law alike?
Boyles Law deals with conditions of constant temperature. Charles' Law deals with conditions of constant pressure. From the ideal gas law of PV = nRT, when temperature is constant (Boyles Law), this can be rearranged to P1V1 = P2V2 (assuming constant number of moles of gas). When pressure is constant, it can be rearranged to V1/T1 = V2/T2 (assuming constant number of moles of gas).
How does the boyles law and Charles law relat to popcorn?
The kinetic and potential energy stored in the corn.
Is airplane an example of boyles law?
yes im not sure why, but yea
When does Boyle's law happen?
Boyles law "happens" when the temperature is held constant and the volume and pressure change.

What is the mathematical expression for Boyles Law?
so the stundent can learn more about math.
A graph of boyles law shows the relationship between?
a graph law graph shows the relationship between pressure and volume
Top Categories

Servicios Personalizados
Indicadores, links relacionados.
Revista mexicana de física E
Versión impresa issn 1870-3542, rev. mex. fís. e vol.64 no.1 méxico ene./jun. 2018.
Evaluation of experimental errors in Boyle’s experiment
E. Ruiz Morillas a
a Repsol Refinery, Industrial Complex of Tarragona, Laboratory, P.O. Box 472, 43080 Tarragona, Spain. e-mail: [email protected]
In this article an analysis of original historical data is made in order to evaluate the experimental errors in Boyle’s experiment. In this evaluation, statistical regression analysis is used to estimate the constant of Boyle’s law and its uncertainty. Also is used the ideal gas law, which was established much later, as a way to evaluate this uncertainty.
This article may be useful to teachers and students as an example of using historical data in physics, and how statistical analysis can be applied to obtain information from these data.
Keywords: Boyle’s law; ideal gas; statistical regression analysis
PACS: 01.30.1b; 01.40.gb
1. Introduction
In 1662 Robert Boyle made several experiments in order to demonstrate that the pressure of a gas and his volume were in reciprocal proportion 1 , 2 , arriving to what today is called Boyle’s law:
P V = K w h e r e K i s a c o n s t a n t (1)
Looking at Eq. (1) the following question can be made: Which is the value of this constant?
This is a question that can be made to the students of a general course of physics for learning the laws of gases. The answer can be obtained from an experimental point of view; to do that, several proposals can be found in the literature that describe Boyle’s law apparatus for a student laboratory and that explain how to use the experimental data to deduce the relationship between volume and pressure of a gas 3 , 4 , 5 . But not always experimentation can be carried out. In this case, it is proposed to recover the historical data of the experiments that led or contributed to the laws that nowadays are studied, to obtain valuable information from them. Such data was obtained in many cases with a lot of effort and great precision 6 . In this article, an answer to the previous question will be proposed through the analysis of the original data obtained by Boyle in the experiment that he made in 1662.
2. Boyle’s experiment
A very detailed description of the historical experiment that led to Boyle’s law can be found in the Part II, chapter V of the Boyle’s book published in 1662 6 , and also in the literature 1 , 2 .
Boyle used a glass tube bended in an U shape having one short leg hermetically closed in its top part and containing a fixed quantity of air in it. He poured mercury into the long leg to compress the air in the short leg. Both legs had scales in inches to measure the height of the cylinder of air in the short leg and the height of the cylinder of mercury in the long leg that was compressing the air.
In the description that Boyle made of the experiment, two parts can be distinguished; in the first part is described the way employed in obtaining an equilibrium state in which there was no height of mercurial cylinder compressing the air, but only the atmospheric pressure. In the second part is described the way of pouring mercury into the longer tube and the way of taking experimental data, as Boyle explains in his book (6):
Then Quicksilver being poured in to fill up the bended part of the Glass, that the surface of it in either leg might rest in the same Horizontal line, as we lately taught, there was more and more Quicksilver poured into the longer Tube; and notice being watchfully taken how far the mercury was raisen in that longer Tube, when it appeared to have ascended to any of the divisions in the shorter Tube. Thus successively made, and as they were made set down, afforded us the ensuing Table .
The data of original Boyle’s experiment is presented in Table I .
Table I. Original Boyle’s experiment data.
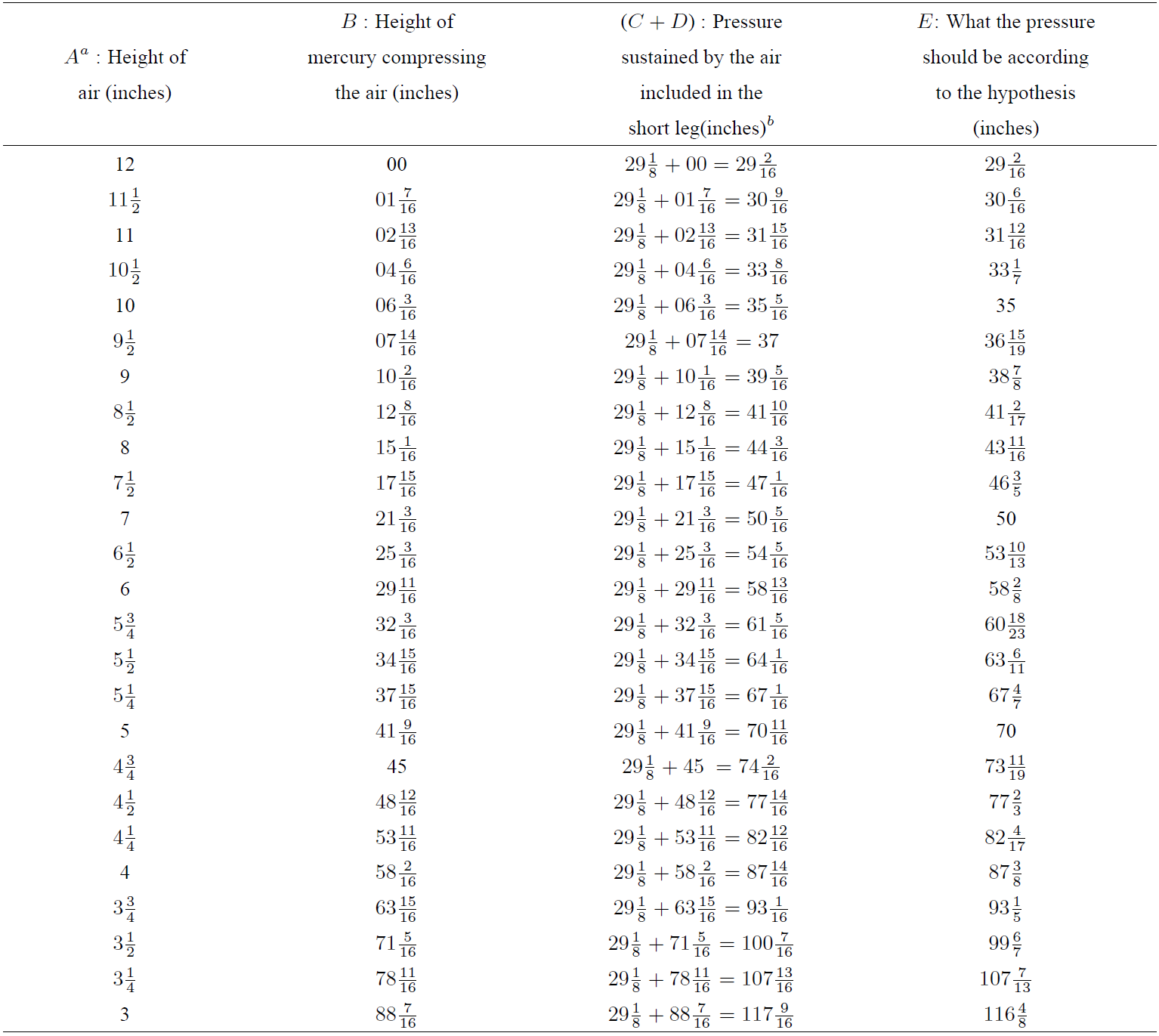
a The letters A to E are the same that are written in the original Table of Boyle’s book 6 to identify the data columns.
b Boyle recorded in his experiment that the height of a mercurial cylinder that counterbalanced the pressure of the atmosphere was 29(1/8) inches; so, he added the value of 29(1/8) inches to every height observed in the longer leg in order to obtain the pressure sustained by the air, and this sum has been explicitally reflected in this table.
According to these results, Boyle concluded that his experiment provides sufficient proves about the reciprocal relationship between the volume and the pressure of the air: when volume of air is reduced to half its initial value, the pressure is near twice it was before; and when the volume is reduced again to half the previous value, the pressure is four times stronger than the initial pressure, although there is a positive bias between experimental pressure values and expected pressure values according to this hypotheses, as it can be seen in Table I . In Fig. 1 it is represented the evolution of this bias as a percentage of the expected pressure value when air is compressed from its initial equilibrium state.
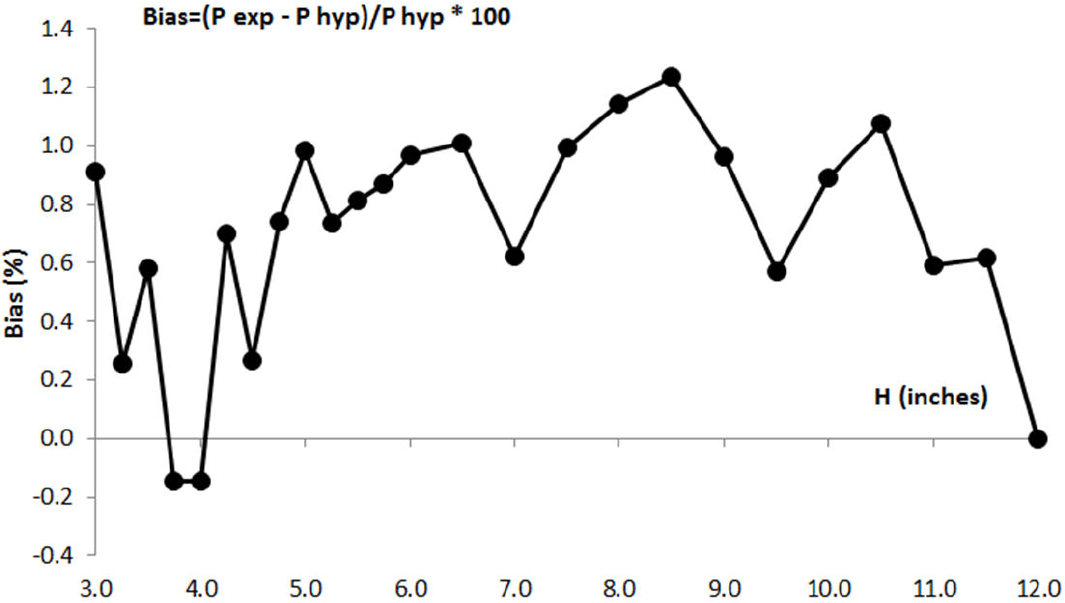
Figure 1. Bias of the experimental pressure in Boyle’s experiment vs H air.
3. Regression analysis of Boyle’s experiment data
Regression analysis is a powerful statistical tool to investigate the relationships between experimental data 2 , 3 , 7 . See Annex about teorical bases of linear regression model at the end of this article.
If we do a regression analysis of the Boyle’s experimental data shown in Table I 7 , the following linear relationship can be obtained:
P ( i n c h e s ) = 350.47 1 H ( i n c h e s ) + 0.2223 (2)
with a determination coefficient R 2 = 0.9999 So, doing this regression analysis it is confirmed the reciprocal relationship explained by Boyle in his book between the volume, expressed in terms of height of air, and the pressure of the air 6 . We have to take into account that the intercept obtained is an estimation of the intercept of the regression model, and an hypothesis test on the intercept shows that there is no statistical differences between the value obtained and zero 7 :
Test on the intercept
β ^ 0 = 0.2223
The value of the statistical t for the intercept is t = 1.599.
The critical value of the t-distribution for a 0.05 significance level and (25 - 2) degrees of freedom is t 0.025,23 = 2.069.
As 1.599 < 2.069 the null hypothesis for the intercept is accepted. So, there is no statistical difference between the value of the intercept of the regression equation and zero.
We can say then, that Eq. (2) is consistent with Eq. (1), taking into account that in Eq. (2) the volume is expressed in terms of height of air.
Analysis of the residuals gives a measure of the variability not explained by the regression model and residuals can be interpreted as the observed values of the errors 8 . The residual plot presented in Fig. 2 shows another way of looking at the evolution of the experimental error in Boyle’s experiment, based on the regression analysis information. We can observe two zones; the first zone goes from the initial point to the point when the volume of air is reduced aproximately to half its initial value. In this zone it can be seen a pattern of autocorrelation 8 . This can be caused by a presence of correlative error in the graduation marks of the tube and has been analyzed in the bibliography 9 . The second zone shows an increase of the experimental error variability when the volume of air is reduced beyond half its initial value. The pattern of this second zone can be situated in an horizontal band between ±0.6 inches, showing a random experimental error that could have different origins; not controlled changes in temperature during experiment or some kind of not controlled practical aspects in measuring at the highest values of pressure.
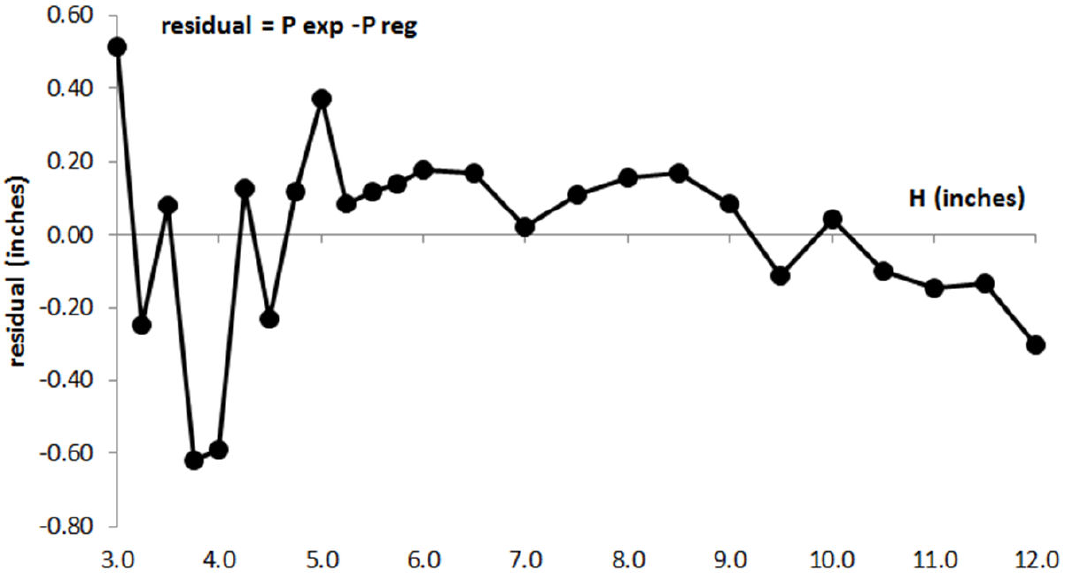
Figure 2. Residual plot in Boyle’s experiment: (experimental pressure-pressure from regression model) vs H air.
Under the assumptions of this regression analysis, from residuals knowledge we can do an estimation of the standard deviation of the experimental error:
σ ^ = 0.26 i n c h e s
and we can stablish a confidence interval for the estimation of the slope of the regresion model, that is; for the estimation of the constant for the reciprocal relationship between the volume of air (in terms of height of air) and the pressure (in terms of height of mercury) at the conditions of the experiment:
K = ( 350.47 ± 1.52 ) i n c h e s 2
That means that the K value found has a relative uncertainty of 0.4%.
4. Discussion of results
In 1662 Robert Boyle made the experiment the results of which have been analyzed. Aproximately 200 years later, in the XIX Century it was established the ideal gas equation 10 :
P V = n R T (3)
where: n : number of moles of gas R : universal gas constant T : absolute temperature of the gas
As we can see comparing Eqs. (1) and (3), the constant of the Boyle’s law is related to the universal gas constant R : The constant of the Boyle’s law is the value of R modified by the product of the number of moles n and the temperature T of the gas, at ( nT ) constant. So, we can write:
K = ( n T ) R w h e r e ( n T ) i s a c o n s t a n t (4)
Therefore, a relative uncertainty of 0.4% in the value of K means a relative uncertainty of 0.4% in the value of R obtained in a reproduction of the Boyle experiment with ( nT ) known, constant and with negligible uncertainty.
As the accepted value of R 11 is:
R = 0.08206 a t m L m o l K
this means that the uncertainty in R will be ±3·10 -4 ; affecting the fourth decimal number of R. This provides a prove about the great precision of the experiment that Boyle made in 1662.
5. Conclusions
An analysis of the original data obtained by Boyle in the experiment that he made in 1662 on the spring of air shows that, although there is a positive bias between the experimental pressure of the air and what the pressure should be according the hypothesis of reciprocal relationship between the volume and the pressure of air, the experimental error is low enough to estimate the constant of Boyle’s law with a relative uncertainty of 0.4%.
This means that a reproduction of Boyle’s experiment with ( nT ) known, allows to obtain from the ideal gas law an estimation of the universal gas constant with uncertainty in the fourth decimal of R , what indicates that Boyle made his experiment with a high degree of precision.
Acknowledgments
Prof. Anna Maria Masdeu-Bultó of the Physical and Inorganic Chemistry Dept. at the University Rovira i Virgili, Tarragona (Spain) is acknowledged for her support, help and suggestions during the redaction of this article.
1. R.G. Neville, J. Chem. Educ. 39 (1962) 356. [ Links ]
2. J.B. West, J. Appl. Phisiol 87 (1999) 1543. [ Links ]
3. D.W. Carter, J. Chem. Educ. 62 (1985) 497. [ Links ]
4. D. McGregor, V. Sweeney, P. Mills, J. Chem. Educ. 89 (2012) 509. [ Links ]
5. W.J. Deal, J. Chem. Educ. 52 (1975) 405. [ Links ]
6. R. Boyle, A Defence of the Doctrine touching the Spring and Weight of the Air , (London, 1662). pp. 57-64. [ Links ]
7. D.C. Montgomery, E.A. Peck, Introduction to Linear Regression Analysis , 2nd Ed.(John Wiley & Sons, New York, 1992). pp. 9-41. [ Links ]
8. D.C. Montgomery, E.A. Peck, Introduction to Linear Regression Analysis , 2nd Ed.(John Wiley & Sons, New York , 1992). pp. 67-79. [ Links ]
9. J. Mandel, The Statistical Analysis of Experimental Data , (John Wiley & Sons, New York , 1964). pp. 295-303. [ Links ]
10. W.B. Jensen, J. Chem. Educ. 80 (2003) 731. [ Links ]
11. R.C. Weast, CRC Handbook of Chemistry and Physics , 66th Ed.(CRC Press, Inc., Boca Raton, Florida, 1985). p. F-196. [ Links ]
12. R.E. Walpole, R.H. Myers, S.L. Myers, K. Ye, Probability & Statistics for Engineers & Scientists 9th Ed. (Prentice Hall, Boston, 2012). pp. 322-323. [ Links ]
13. D.C. Montgomery, E.A. Peck, Introduction to Linear Regression Analysis , 2nd Ed. (John Wiley & Sons, New York , 1992). Table A-3 p 467. [ Links ]
A. Teorical bases of linear regression model
To express the relationship between two variables, we can use the following linear regression model 7 :
y = β 0 + β 1 x + ε (5)
x represents the values of the independent variable. In an experiment, this is the variable controlled by the experimenter.
y represents the values of the dependent variable. In an experiment, these are the results of the experiment for each value of the independent variable.
ε is the aleatory component of the values of the dependent variable due to the experimental error, and it is considered that it has a normal distribution N ( 0 , σ ) .
An estimation of this linear regression model can be made from a given set of experimental data using the least squares method:
y ^ = β ^ 0 + β ^ 1 x
where, given the n pairs of data ( x 1 , y 1 ), ( x 2 , y 2 ), …, ( x n , y n ):
β ^ 1 = S x y S x x β ^ 0 = y ¯ - β ^ 1 x ¯
In this expressions, the following notation has been introduced to express sumatories:
S x x = ∑ i = 1 n ( x i - x ¯ ) 2 S x y = ∑ i = 1 n y i ( x i - x ¯ ) x ¯ = 1 n ∑ i = 1 n x i y ¯ = 1 n ∑ i = 1 n y i
The residual e i is the difference between the yi value obtained in the experiment and the corresponding adjusted y ^ i value obtained from the regression equation:
e i = y i - y ^ i = y i - ( β ^ 0 + β ^ 1 x i ) i = 1,2 , … , n
The parameter MS E is an estimation of the experimental error variance σ 2 and its expression is:
σ ^ 2 = M S E = 1 n - 2 ∑ i = 1 n e i 2
An estimation of the standard deviation of experimental error σ is then:
σ ^ = M S E
An hypothesis testing on the intercept of the linear regression model can be done to know if it is equal to a given value b:
H 0 : β 0 = b H 1 : β 0 ≠ b
using the statistical t for the intercept whose possible values are:
t = β ^ 0 - b M S E ( 1 n + x ¯ 2 S x x )
and it has a t-student distribution with ( n - 2)degrees of fredom. For an α significance level 12 , H 0 is accepted if - t ( α / 2 ) , n - 2 ≤ t ≤ t ( α / 2 ) , n - 2 where t ( α / 2 ) , n - 2 is the upper α / 2 percentage point of the t-distribution with ( n - 2) degrees of freedom (also called critical value) 12 , and it is tabulated 13 .
The confidence interval of ( 1 - α )100% for the slope β 1 is:
β ^ 1 - t α 2 , n - 2 M S E S x x < β 1 < β ^ 1 + t α 2 , n - 2 M S E S x x
Received: March 07, 2017; Accepted: September 05, 2017

Newest Articles
- Understanding Diffraction Grating Formula for Physics Enthusiasts
- Understanding Momentum: Everything You Need to Know
- Simulation Software: Unlocking the Wonders of Physics
- Understanding Thermal Equilibrium Problems
- Acceleration
- Electricity and Magnetism
- Electric current
- Electrostatics
- Magnetic fields
- Modern Physics
- Quantum mechanics
- Particle physics
- Thermodynamics
- Temperature
- Heat transfer
- Newton's Laws
- Light waves
- Mirrors and lenses
- Interference and diffraction
- Kinematics formulas
- Velocity formula
- Acceleration formula
- Displacement formula
- Dynamics formulas
- Newton's Second Law formula
- Force formula
- Momentum formula
- Electricity and Magnetism formulas
- Coulomb's Law formula
- Ohm's Law formula
- Magnetic force formula
- Thermodynamics formulas
- Heat capacity formula
- Ideal gas law formula
- Entropy formula
- Optics formulas
- Snell's Law formula
- Diffraction grating formula
- Lens formula
- Modern Physics formulas
- Higgs boson mass formula
- Schrodinger equation formula
- E=mc^2 formula
- Thermodynamics experiments
- Heat transfer experiment
- Boyle's Law experiment
- Carnot cycle experiment
- Classical Mechanics experiments
- Conservation of energy experiment
- Newton's Cradle experiment
- Projectile motion experiment
- Modern Physics experiments
- Quantum entanglement experiment
- Particle accelerator experiment
- Photoelectric effect experiment
- Electricity and Magnetism experiments
- Magnetic field mapping experiment
- Electric field mapping experiment
- Ohm's Law experiment
- Optics experiments
- Diffraction grating experiment
- Double-slit experiment
- Polarization experiment
- Dynamics problems
- Friction problems
- Circular motion problems
- Momentum conservation problems
- Electricity and Magnetism problems
- Electric field problems
- Magnetic force problems
- Circuit analysis problems
- Kinematics problems
- Displacement problems
- Velocity problems
- Acceleration problems
- Thermodynamics problems
- Thermal equilibrium problems
- Entropy change problems
- Heat transfer problems
- Optics problems
- Lens equation problems
- Snell's Law problems
- Diffraction grating problems
- Modern Physics problems
- Wave-particle duality problems
- Schrodinger equation problems
- Special relativity problems
- Thermodynamics tutorials
- Heat transfer tutorial
- Temperature tutorial
- Entropy tutorial
- Dynamics tutorials
- Momentum tutorial
- Force tutorial
- Work and energy tutorial
- Kinematics tutorials
- Velocity tutorial
- Acceleration tutorial
- Displacement tutorial
- Electricity and Magnetism tutorials
- Electric field tutorial
- Magnetic field tutorial
- Circuit analysis tutorial
- Optics tutorials
- Reflection and refraction tutorial
- Mirrors and lenses tutorial
- Interference and diffraction tutorial
- Modern Physics tutorials
- Relativity tutorial
- Particle physics tutorial
- Quantum mechanics tutorial
- Reference materials
- Equation sheets
- Formula calculators
- Online resources
- Physics websites
- Online courses
- Videos and tutorials
- Laboratory equipment
- Simulation software
- Experiment kits
- Measurement tools
- Books and textbooks
- Study guides and problem sets
- Advanced physics textbooks
- Introductory physics books
- Undergraduate physics education
- Degree programs
- Coursework requirements
- Research opportunities
- High school physics education
- Extracurricular activities
- Curriculum standards
- Teaching resources
- Graduate physics education
- Master's programs
- Thesis and dissertation requirements
- PhD programs
- Academic careers in physics
- Research positions
- Professorship positions
- Teaching positions
- Industry careers in physics
- Engineering jobs
- Data analysis jobs
- Consulting positions
- Government and non-profit careers in physics
- Museum and outreach positions
- Policy and advocacy jobs
- National laboratory positions
- Classical Mechanics research
- Celestial mechanics
- Nonlinear dynamics
- Fluid mechanics
- Thermodynamics research
- Statistical mechanics
- Heat engines
- Phase transitions
- Electricity and Magnetism research
- Electromagnetism
- Plasma physics
- Quantum electrodynamics
- Optics research
- Fiber optics
- Nonlinear optics
- Quantum optics
- Modern Physics research
- Quantum computing
- Understanding Boyle's Law Experiment
- Physics experiments
In the world of physics, there are countless experiments that have helped us understand the laws of nature. One such experiment is Boyle's Law experiment, named after the Irish scientist Robert Boyle who conducted it in the 17th century. This experiment is a fundamental concept in thermodynamics and is essential for understanding the behavior of gases. It involves studying the relationship between the pressure and volume of a gas at constant temperature.
The results of this experiment have been crucial in advancing our understanding of how gases behave and have practical applications in various fields, from scuba diving to medical technology. In this article, we will delve deeper into Boyle's Law experiment, exploring its history, significance, and real-life applications. So, let's begin our journey into the fascinating world of physics experiments with a focus on thermodynamics. First, let's start with the basics. Boyle's Law is a gas law that describes the relationship between pressure and volume at a constant temperature.
It states that as the pressure of a gas increases, its volume decreases, and vice versa. This law was discovered by Irish chemist and physicist Robert Boyle in the 17th century and has since been a crucial concept in understanding the behavior of gases. Boyle's Law experiment is a simple yet effective way to demonstrate this law. The experiment involves a closed system with a fixed amount of gas at a constant temperature. By changing the pressure of the gas and measuring its corresponding volume, we can observe the inverse relationship between the two variables. One popular example of this experiment is using a syringe filled with air.
As we push down on the plunger, the pressure inside the syringe increases, causing the volume of air to decrease. This is because the increased pressure compresses the gas molecules, reducing the space they occupy. Another way to visualize Boyle's Law is by using a graph of pressure versus volume. The resulting curve is a hyperbola, with pressure and volume having an inverse relationship. This graph can also be used to calculate the constant value in Boyle's Law equation, PV = k.Now, you may be wondering why Boyle's Law is so important.
Well, it has many practical applications in our daily lives. For example, it helps explain how a balloon expands when we blow air into it, or how scuba divers use compressed air tanks to breathe underwater. In the field of thermodynamics, Boyle's Law is essential for understanding the behavior of gases in different systems. It also serves as a fundamental principle for other gas laws such as Charles' Law and Gay-Lussac's Law. In conclusion, Boyle's Law experiment is a crucial part of understanding the behavior of gases in various systems. Whether you are a student, researcher, or simply curious about physics experiments, this law is a fundamental concept that is worth exploring.
Understanding Boyle's Law
Conducting a boyle's law experiment, helpful tutorials and resources, careers in physics.
Whether you are interested in conducting research, teaching, or working in industry, a strong foundation in Boyle's Law will be essential in your career. Some of the fields of physics that you can pursue include astrophysics, particle physics, biophysics, and many more. Each field offers unique challenges and opportunities to contribute to our understanding of the universe. As a physicist, you can also work in a variety of industries such as aerospace, energy, and technology. These industries rely on the principles of physics to develop new technologies and improve existing ones. With the rapid advancements in technology, there is a high demand for skilled physicists in the job market. To get started on your career path in physics, it is important to have a strong understanding of fundamental concepts like Boyle's Law.
Solving Problems Using Boyle's Law
For example, if you have a fixed amount of gas in a container and you increase the pressure, the volume of the gas will decrease proportionally according to Boyle's Law. This relationship can be expressed mathematically as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume. Another application of Boyle's Law is in determining the pressure of a gas at different volumes. For instance, if you have a gas in a container with a fixed volume and you decrease the volume, the pressure will increase according to Boyle's Law. This can be represented as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume. By understanding how to apply Boyle's Law, you can solve various problems involving gases and their properties.
Latest Research in Boyle's Law
It states that as the volume of a gas decreases, the pressure increases proportionally. This law has numerous applications in industries such as chemistry, engineering, and medicine. In recent years, there have been several advancements in understanding Boyle's Law and its applications. One study published in the Journal of Chemical Education explored the use of Boyle's Law in determining the amount of carbon dioxide in soft drinks.
This research has implications for quality control in the beverage industry. Another study published in Physical Review Letters investigated the effects of Boyle's Law on quantum gases. The researchers found that at ultra-low temperatures, gases behave differently than predicted by classical Boyle's Law. This discovery has opened up new possibilities for understanding the behavior of matter at extremely low temperatures.
In recent years, there have been numerous studies conducted on Boyle's Law and its applications in various fields. Researchers have been able to apply this fundamental concept in thermodynamics to solve real-world problems and make significant advancements in different industries. One area where Boyle's Law has been extensively studied is in the field of gas dynamics. Scientists have been able to use this law to predict the behavior of gases at different temperatures and pressures, which has led to the development of more efficient engines and turbines. Another interesting application of Boyle's Law is in the medical field. By understanding how gases behave under different conditions, researchers have been able to develop better respiratory equipment and treatments for patients with respiratory illnesses. Furthermore, research on Boyle's Law has also led to a better understanding of the behavior of fluids in general.
Example Problem:
This includes the properties of gases and how they behave under different conditions. In simple terms, Boyle's Law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. This means that if the volume of a gas decreases, its pressure will increase and vice versa. This law was first discovered by Irish scientist Robert Boyle in the 17th century during his experiments with air and the properties of gases.
In order to understand this law in depth, we must first understand the properties of gases. Unlike solids and liquids, gases have no definite shape or volume. They are able to expand and contract to fill the space available to them. The behavior of gases is governed by various physical laws and principles, one of which is Boyle's Law.
Now, let us delve deeper into the concept of pressure and volume in relation to gases. Pressure refers to the force exerted by a gas on the walls of its container. This force is a result of the collisions between gas molecules and the container walls. The volume of a gas, on the other hand, refers to the amount of space it occupies.
When we apply Boyle's Law to these two variables, we can see how they are inversely related. As the volume of a gas decreases, its molecules are pushed closer together, resulting in more frequent collisions with the container walls and therefore, an increase in pressure. Similarly, when the volume increases, there is more space for the molecules to move around, leading to fewer collisions and a decrease in pressure. Understanding these basic principles is crucial in comprehending Boyle's Law and its implications in thermodynamics experiments.
Another research
A more recent study.
Boyle's Law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. In simpler terms, as pressure increases, the volume decreases and vice versa. This means that we can use this relationship to solve for unknown variables in problems involving gases. For example, if we know the initial volume and pressure of a gas and want to find the final volume after a change in pressure, we can use Boyle's Law to calculate it.
Another practical application of Boyle's Law is in experiments involving gases. By manipulating the pressure and volume of a gas, we can observe the effects on other properties such as temperature and mass. This helps us understand the behavior of gases and their properties. Overall, understanding Boyle's Law is crucial for solving problems and conducting experiments related to gases and thermodynamics.
So whether you are a student, researcher, or simply curious about the world of physics, make sure to keep this law in mind!By now, you should have a solid understanding of Boyle's Law and how it relates to thermodynamics . Whether you are conducting an experiment or using it to solve problems, this law is a fundamental concept that is crucial to grasp. Keep exploring and learning about the exciting world of physics !.
- A Comprehensive Guide to Understanding Displacement in Physics
- Exploring Celestial Mechanics: Understanding the Complexities of Classical Mechanics
- Understanding Coursework Requirements for Physics Education
- Exploring Projectile Motion: A Comprehensive Guide to Understanding and Conducting Experiments
- Understanding the Newton's Cradle Experiment
- A Comprehensive Look at Physics Degree Programs
- Understanding Wave-Particle Duality: Exploring the Dual Nature of Light and Matter
- Exploring Friction Problems
- A Beginner's Guide to Particle Physics
- A Comprehensive Guide to Physics Study Guides and Problem Sets
- Exploring Ohm's Law: Understanding the Relationship Between Voltage, Current, and Resistance
- Exploring the Displacement Formula: A Comprehensive Guide to Understanding Kinematics
- Exploring Professorship Positions in the World of Physics
- Exploring the World of Advanced Physics Textbooks
- Exploring the Fascinating World of Light Waves
- Understanding Position in Physics: A Comprehensive Guide
- Understanding the E=mc^2 Formula: A Comprehensive Look into the Famous Equation
- Understanding Thesis and Dissertation Requirements for Graduate Physics Education
- Understanding Statistical Mechanics: Exploring the Fundamentals of Thermodynamics and Physics
- Accelerate Your Understanding: A Comprehensive Tutorial on Physics Concepts and Formulas
- Understanding Electric Current: An In-Depth Look
- A Comprehensive Guide to Experiment Kits for Physics Enthusiasts
- A Comprehensive Overview of Formula Calculators in Physics
- A Comprehensive Guide to Introductory Physics Books
- Circular Motion Problems: A Comprehensive Guide
- A Comprehensive Guide to Equation Sheets in Physics
Understanding Nonlinear Optics: Exploring the Fascinating World of Light and Matter
- Understanding the Photoelectric Effect Experiment
- Understanding Temperature: A Comprehensive Guide to Physics and Thermodynamics
- Understanding Velocity in Physics: Everything You Need to Know
- A Beginner's Guide to Understanding Velocity in Physics
- Electromagnetism: Exploring the Powerful Connection Between Electricity and Magnetism
- Understanding the Velocity Formula
Understanding Quantum Electrodynamics: A Comprehensive Overview
- A Comprehensive Guide to Engineering Jobs
- Exploring Careers in Data Analysis
- An Introduction to Reflection and Refraction in Physics
- Master's Programs in Physics: A Comprehensive Guide
- An Introduction to Work and Energy in Physics
- Understanding Glossaries for Physics: A Comprehensive Guide
- Understanding the Force Formula in Physics
- Understanding Newton's Second Law Formula
- Understanding Snell's Law Formula: A Comprehensive Guide to Optics Formulas
- A Comprehensive Guide to Physics Websites: Everything You Need to Know
- Understanding Coulomb's Law Formula
- Understanding Magnetic Force Formula
- Exploring the World of Physics: A Comprehensive Guide to Research Opportunities
- A Comprehensive Guide to Consulting Positions in the Physics Industry
- Understanding Electric Field Mapping Experiment
- Exploring Electric Field Problems
- Exploring the Power of Force
- Exploring the Wonders of Magnetic Fields
- All You Need to Know About Videos and Tutorials for Learning Physics
- Understanding the Lens Formula: A Comprehensive Guide to Optics Formulas
- Understanding Plasma Physics: Exploring Concepts, Formulas, Experiments, and Careers
- Momentum Tutorial: Understanding Physics Concepts and Formulas
- Exploring Careers in Physics: A Guide to Policy and Advocacy Jobs
- Exploring the Wonders of Fiber Optics: A Comprehensive Introduction
- A Comprehensive Overview of Relativity
- Understanding Heat Transfer Problems
- Understanding Interference and Diffraction in Optics
- Understanding Cosmology: Exploring the Mysteries of the Universe
- Understanding Ohm's Law Formula
- The Basics of Force: Understanding Physics Concepts and Applications
Exploring the World of Physics Through Online Courses
- Understanding the Schrodinger Equation Formula
- Unlocking the World of Physics: A Comprehensive Guide to Extracurricular Activities
- Understanding the Entropy Formula
- Understanding Entropy: A Comprehensive Guide to the Physics Concept of Disorder
- Understanding Heat Transfer: A Comprehensive Guide
- Understanding Momentum Conservation Problems
- Exploring the Fascinating World of Particle Physics
- Understanding the Polarization Experiment
- Exploring the World of Particle Physics
- Understanding Acceleration: A Comprehensive Guide to Physics Concepts and Formulas
- Understanding Schrodinger Equation Problems
- Understanding Special Relativity Problems
- Understanding Lens Equation Problems: A Comprehensive Guide
- Understanding Electrostatics: A Comprehensive Overview
- Understanding the Momentum Formula: An Essential Guide for Physics Enthusiasts
- Exploring Magnetic Field Mapping: A Comprehensive Overview
- Understanding Mirrors and Lenses: A Comprehensive Guide to Physics Concepts in Optics
- Covering all aspects of teaching resources in physics education
- Understanding Displacement Problems in Physics
- Understanding Curriculum Standards in Physics Education
- An Introduction to Entropy: Understanding Physics Concepts and Formulas
- Exploring Quantum Entanglement: Understanding the Concept and Conducting Experiments
- Electric Field Tutorial: Understanding the Principles of Electricity and Magnetism
- Understanding Quantum Optics: A Comprehensive Overview
- A Comprehensive Guide to Circuit Analysis Problems
- Understanding Diffraction Grating Experiment
- Understanding Magnetic Force Problems
- Exploring National Laboratory Positions in the Field of Physics
- Understanding Acceleration: A Comprehensive Guide
- A Comprehensive Look at Fluid Mechanics
Recent Articles

Which cookies do you want to accept?

IMAGES
COMMENTS
ev alua tion of experiment al errors in boyle's experiment 45 Therefore, a relative uncertainty of 0.4% in the value of K means a relative uncertainty of 0.4% in the value of R ob-
obtained by Boyle in the experiment that he made in 1662. 2. Boyle's experiment A very detailed description of the historical experiment that led to Boyle's law can be found in the Part II, chapter V of the Boyle's book published in 1662 [6], and also in the liter-ature [1,2]. Boyle used a glass tube bended in an U shape having one
Boyle's Law relates gas pressure (P) to volume (V) by the equation P1V1 = P2V2. ... Possible sources of errors in Boyle's law experiments include temperature changes affecting gas volume ...
#ñÿ ¢ G¤&õh„ ŸóþÛ›Öÿwߟ/Š©©$õŒ$Ðî ûÝ$ ^³l½Í[($Ž$ ËîÅÿÿýz•e iJ è».ÏF\ ˜ç·|ï¹÷¼7 à §ˆ"_tU•]>È2ª`kÄì7fÖ+ ŒŒ ...
2. Boyle's experiment. A very detailed description of the historical experiment that led to Boyle's law can be found in the Part II, chapter V of the Boyle's book published in 1662 6, and also in the literature 1, 2.. Boyle used a glass tube bended in an U shape having one short leg hermetically closed in its top part and containing a fixed quantity of air in it.
A recent article1 in The Physics Teacher describes a method for analyzing a systematic error in a Boyle's law laboratory activity. Systematic errors are importa
Here is the sequence of three Chemistry Applet webpages mentioned in the Introduction. These will really help your understanding of Boyle's Law if you take the time to do the virtual experiments! Blauch, D., (2004). Gas Laws: Pressure. Department of Chemistry, Davidson College. Retrieved October 19, 2023. Blauch, D., (2004). Gas Laws: Boyle's ...
experiment. 2 pts It is evident that the student was able to connect the learning goals of the experiment with data obtained in the experiment. Quality of your writing 2 pts It is written in complete sentence(s). 2 pts The sentences are comprehendible to the reader. 2 pts It summarizes the experiment and the result and puts the results in context
For verifying Boyle's Law, measuring pressure in mm of Mercury (Torr) and volume in units of the length of the closed end of the tube is convenient. You will convert your final value of pV to the SI unit of newton-meters = joules .
Boyle's Law experiment is a simple yet effective way to demonstrate this law. The experiment involves a closed system with a fixed amount of gas at a constant temperature. By changing the pressure of the gas and measuring its corresponding volume, we can observe the inverse relationship between the two variables. One popular example of this ...